The genus trace: a function that shows values of genus (vertical axis) for subchains spanned between the first residue, and all other residues (shown on horizontal axis). The number of the latter residue and the genus of a given subchain are shown interactively.
Total Genus |
71
|
sequence length |
263
|
structure length |
258
|
Chain Sequence |
HMQAEILLTLKLQQKLFADPRRISLLKHIALSGSISQGAKDAGISYKSAWDAINEMNQLSEHILVERATGGAVLTRYGQRLIQLYDLLAQIQQKAFDVLSDDDALPLNSLLAAISRFSLQTSARNQWFGTITARDHDDVQQHVDVLLADGKTRLKVAITAQSGARLGLDEGKEVLILLKAPWVGITQDEAVAQNADNQLPGIISHIERGAEQCEVLMALPDGQTLCATVPVNEATSLQQGQNVTAYFNADSVIIATLC
|
The genus matrix. At position (x,y) a genus value for a subchain spanned between x’th and y’th residue is shown. Values of the genus are represented by color, according to the scale given on the right.
After clicking on a point (x,y) in the genus matrix above, a subchain from x to y is shown in color.
| publication title |
The high-resolution crystal structure of the molybdate-dependent transcriptional regulator (ModE) from Escherichia coli: a novel combination of domain folds.
pubmed doi rcsb |
| molecule keywords |
PROTEIN (MODE)
|
| molecule tags |
Transcription
|
| source organism |
Escherichia coli
|
| total genus |
71
|
| structure length |
258
|
| sequence length |
263
|
| chains with identical sequence |
B
|
| ec nomenclature | |
| pdb deposition date | 1999-02-12 |
| chain | Pfam Accession Code | Pfam Family Identifier | Pfam Description |
|---|---|---|---|
| A | PF00126 | HTH_1 | Bacterial regulatory helix-turn-helix protein, lysR family |
| A | PF03459 | TOBE | TOBE domain |
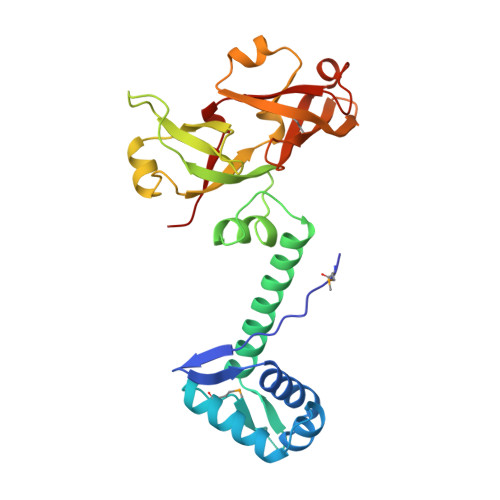
 Image from the rcsb pdb (www.rcsb.org)
Image from the rcsb pdb (www.rcsb.org)
cath code
| Class | Architecture | Topology | Homology | Domain |
|---|---|---|---|---|---|
| Mainly Alpha | Orthogonal Bundle | Arc Repressor Mutant, subunit A | Winged helix-like DNA-binding domain superfamily/Winged helix DNA-binding domain | ||
| Mainly Beta | Beta Barrel | OB fold (Dihydrolipoamide Acetyltransferase, E2P) | OB fold (Dihydrolipoamide Acetyltransferase, E2P) | ||
| Mainly Beta | Beta Barrel | OB fold (Dihydrolipoamide Acetyltransferase, E2P) | OB fold (Dihydrolipoamide Acetyltransferase, E2P) |
#chains in the Genus database with same CATH superfamily 4LG0 B; 4U88 A; 1KU7 A; 5HLH A; 4R79 A; 1TU2 B; 1CTM A; 2LNB A; 1SFU A; 4MTE A; 4KKU A; 1VF5 C; 3IKT A; 1LDJ A; 2EV5 A; 2AS5 F; 3KP7 A; 4HW0 A; 3REO A; 1OXT A; 2VOO A; 3KP2 A; 4OU0 A; 5TRD A; 5JEA G; 4B8X A; 4MQV A; 1DD2 A; 1DPR A; 3GLX A; 5E1X A; 2RQP A; 1ZRC A; 4JBA A; 3FM3 A; 3RJP A; 4CIC A; 2NN6 G; 1OR7 A; 4JK1 C; 3ABT A; 5HLG A; 1F1Z A; 2L54 A; 2JEB I; 2FEZ A; 4IRH A; 2G9W A; 3HRM A; 3CO7 C; 1WQ2 A; 1HKS A; 3SDZ A; 1WI9 A; 2ISZ A; 1CFM A; 1KU3 A; 4AVP A; 4GGG A; 4EGY A; 2DQL A; 2FU4 A; 4MGR A; 1FP1 D; 1IZ1 A; 3WTU C; 1XMK A; 4ZH4 F; 4NHJ A; 1Y69 U; 1HTP A; 2L4M A; 1O3T A; 4KFC A; 3LNN A; 3ECH A; 3CRL C; 2QLZ A; 1GHK A; 3G3Z A; 3IWZ A; 3EDP A; 4UNO A; 1IXS B; 1MKM A; 5FLX Z; 4OIR F; 1E17 A; 1OXS C; 5BPI A; 4I0A A; 1P4X A; 3HTU A; 3HOT A; 1Y8O B; 3GUD A; 1SMY C; 2DPU A; 4U0W A; 1YIO A; 2RC4 A; 2VBY A; 3KLO A; 2LF8 A; 2A6H D; 1IN6 A; 4O3M A; 4EJT A; 5BOX A; 4NB5 A; 3JSO A; 1YNJ C; 3TGN A; 4HF2 A; 3K69 A; 2ZT9 C; 2D1V A; 4PGH A; 3QP5 A; 4OO1 G; 2O3F A; 3NEU A; 4KMU X; 2HYG D; 1HST A; 4A0Y A; 1TQM A; 1ZRE A; 5DDG A; 1J5Y A; 1ZRF A; 4KNY A; 1ZYR C; 3VR2 A; 1T98 A; 1YTY A; 5JI6 A; 1CGP A; 4IFD H; 1ZHF A; 5ILS A; 4RGR A; 3K1P A; 5HDK A; 2O61 A; 2QM3 A; 1BI1 A; 2LF7 A; 4FHT A; 3PFI A; 2DTR A; 4HAM A; 1PMR A; 3VR5 A; 3Q9V A; 2JE6 I; 1ZGA A; 3D0S A; 1QO0 D; 1B9N A; 2A6H C; 1I5Z A; 2D62 A; 4UHT A; 2IW5 A; 3JAM Z; 3KZZ A; 1XCB A; 2EWN A; 5BMZ A; 4XBF A; 4LJZ C; 1OYW A; 2CW0 C; 1O7F A; 2Z99 A; 4LLN A; 2ADU A; 4L8J A; 3BY6 A; 4ZZL A; 1OXU A; 5HS7 A; 5DEQ A; 4KYW A; 1P4A A; 3TQN A; 3G73 A; 1QNT A; 2Z5U A; 2Z33 A; 3L7Z C; 2EB7 A; 3P9K A; 4N9H A; 2VT3 A; 3F8B A; 1YO5 C; 5JR3 A; 2GQQ A; 4P9U A; 5LHG A; 4MEX F; 4UUV A; 4LLL A; 2PLY A; 5D5U B; 2A07 F; 3IKJ A; 1IN5 A; 2FBH A; 3J80 K; 2HKX A; 4CHU A; 1S7A A; 2JPB A; 2BGC A; 1R58 A; 1TBX A; 4BXU A; 1L0O C; 4R8H A; 2QQA A; 2K27 A; 3ECO A; 1EH6 A; 2STT A; 3WTV C; 3V4G A; 5K5R A; 4LK1 C; 3ZMV A; 2EFQ A; 3R4K A; 3GZ6 A; 2B8G A; 2CFX A; 3ZMU A; 2E74 C; 2DAO A; 1IYV A; 1R36 A; 4BNC A; 1RNL A; 5KNB A; 1SMY F; 4QLC U; 3CTA A; 2IA0 A; 3SE0 A; 5HS9 A; 2VOD A; 2EFW A; 2K33 A; 3V8K A; 2HQR A; 1BOA A; 2PJP A; 5LRS A; 3Q5F A; 2FOK A; 3WG9 A; 1UHM A; 2HGC A; 4EJW A; 3WTT C; 1SFE A; 2FNP A; 4OIQ F; 4ON0 A; 2NNN A; 4YFK F; 1ZYR F; 2D2C C; 1YS7 A; 4WK8 F; 1ZN2 A; 1RZ4 A; 1FBU A; 1Z05 A; 1BJA A; 5FO5 A; 3VR4 A; 3E6B A; 3ERE D; 2F5F A; 3KET A; 2A6H F; 2EFN A; 4WF2 A; 4ESJ A; 2BYV E; 4IJA A; 1R22 A; 4DNC A; 4LJZ F; 4OMY A; 4OMZ A; 5FB2 A; 3BWG A; 1QBJ A; 2CW0 F; 1E2X A; 3GQB A; 5DD8 A; 1O3R A; 4K2E A; 4U7D A; 2DNE A; 2PI0 A; 1XCV A; 5A35 A; 4RB2 C; 2V6C A; 3BP8 A; 5ICC A; 2RGV A; 4M73 A; 3WU0 A; 3CJN A; 3F8C A; 5JEA H; 4FX0 A; 1YW7 A; 1Z91 A; 4XSX F; 5K36 G; 3GWZ A; 3J81 Z; 3V8L A; 4CZZ A; 1JGS A; 3KP3 A; 1KU9 A; 4G94 A; 2NN6 H; 1MJB A; 3ISP A; 4FX4 A; 1PDN C; 1Z9C A; 5D4S A; 3WGG A; 3I64 A; 1R23 A; 1HPC A; 3T5X A; 4UV8 A; 1HQC A; 2C6Y A; 3KP5 A; 2JZY A; 1JHF A; 3BRO A; 1IN7 A; 1OPC A; 3A8I E; 2H09 A; 2MA3 A; 3F6O A; 2GAU A; 3M9A A; 1S7O A; 5D8K B; 4AD9 A; 2O5I C; 4L9T A; 4DK1 A; 3ROU A; 2DLL A; 4EVI A; 4XLN F; 2H94 A; 4M72 A; 4G8X A; 3D5L A; 5DUI A; 3T53 B; 2YPR A; 3QP6 A; 4JBW A; 2M8E A; 2J5P A; 1JE8 A; 4LK1 F; 5KVR A; 2DW4 A; 2ZCW A; 2OA4 A; 4I02 A; 1KGS A; 1H9M A; 2YSR A; 5D6F A; 2QF7 A; 4G7Z C; 2W57 A; 3SHF A; 2XAS A; 3Q9S A; 2NAZ A; 1T39 A; 5BQT A; 2KA7 A; 4HLY A; 1P2F A; 2BEO A; 1O7L A; 4I0B A; 5FLX K; 1Z6R A; 4HOB A; 3GFJ A; 2NRA C; 3F8N A; 1RIO H; 1BI0 A; 4BYY A; 2A69 D; 2IPQ X; 1Y8P B; 1LVA A; 3RYR A; 2BBY A; 2PNR C; 5E8I A; 3PUW A; 2FXA A; 4MHG A; 3QG1 A; 1O57 A; 1E2V A; 4IHT A; 1XMA A; 2XKO A; 3BDD A; 3L2C A; 3TKY A; 1W7P D; 4G7H C; 2BA1 A; 1D3Y A; 2H6C A; 1IXC A; 3M7N A; 1G3Y A; 1CF7 A; 1XGO A; 1CF7 B; 4YFN F; 1V4R A; 2BV6 A; 1WSU A; 4KUM A; 1TLH B; 1FYL A; 2QQ9 A; 1E2W A; 5IT9 K; 4A0Z A; 1ZAR A; 4MNU A; 4IF4 A; 1TQP A; 1MJ9 A; 4KN7 C; 4BA1 I; 2Z9O A; 4LB6 B; 1IAW A; 2ISY A; 2JPC A; 1VF7 A; 2OAZ A; 4IRG A; 1FYM A; 2A69 C; 3HRT A; 1ODD A; 3CUQ A; 3FHZ A; 3JAM K; 3CUQ B; 1DP7 P; 3I71 A; 4IQZ A; 5CVI A; 2VE9 A; 5LGN A; 4ASN A; 1U8R A; 1YW9 A; 2Q0O A; 4EV0 A; 4I7H A; 4GYI A; 3PQK A; 3ZP5 A; 4LB5 A; 3OV8 A; 2VXZ A; 2D45 A; 1QGP A; 3LWF A; 4RB0 A; 1P6R A; 2KIU A; 3BJO A; 1ZG3 A; 2X4H A; 4KN4 C; 3TOA A; 5DV5 A; 3FMC A; 4RS8 A; 3A8K E; 2V79 A; 3U9S A; 5ICE A; 1ULY A; 5F6F A; 4M74 A; 2EFO A; 2AWO A; 5A2Q Z; 3T56 B; 1VDZ A; 3LQR A; 4MEY F; 5L3C A; 4KKT A; 1KA8 A; 1MD0 A; 1KYW A; 4BA2 I; 2XGX A; 3E5U A; 1FBS A; 4A0X A; 4KA4 A; 1I6X A; 2UWM A; 2CO5 A; 4PCQ A; 4G7Z F; 1KYZ A; 3HYI A; 2Q8K A; 4KIG A; 3FMS A; 2P6T A; 3E6D A; 1MDM A; 1ZLK A; 3U1D A; 5F64 A; 1HKT A; 2QQB A; 1FT9 A; 1MDM B; 4RAY A; 1SD4 A; 2MBF A; 3FM5 A; 1LDK B; 1W7P A; 5LRR A; 3LA2 A; 1FC3 A; 2K4B A; 1W7P B; 2VC1 A; 3F72 A; 4M9S A; 1S6L A; 5LHH A; 1FLI A; 3LAP A; 2EJF C; 3MQ0 A; 1FYK A; 5L3B A; 2JEA I; 2FBI A; 1IYU A; 2HDC A; 5CVR A; 3V7R A; 3ABU A; 1O78 A; 4DK0 A; 2AXL A; 4G7H F; 4G6Q A; 4I01 A; 3C1D A; 4A6D A; 3ZPL A; 2M87 A; 4YIF A; 2P6S A; 3DXJ C; 3F6V A; 4EQQ A; 3ZMT A; 1K7A A; 3CLO A; 2P5K A; 1IXR C; 2H27 A; 2GIV A; 4GZY C; 3GX4 X; 1LDD A; 5BN5 A; 1U6G A; 3MZY A; 5IT3 A; 2FSW A; 6PAX A; 5FD5 A; 2FF4 A; 5D6E A; 1EWH A; 4NQW A; 2K7V A; 4ETS A; 5K5Q A; 3LA3 A; 1R5H A; 2A69 F; 4A5N A; 1LAB A; 2BE5 C; 1HXD A; 1F4K A; 4S04 A; 5ICG A; 1IN8 A; 2E7W A; 2P7C B; 4H44 C; 2ZME A; 2YR2 A; 2ZME B; 1ONV A; 2G7H A; 5BS6 A; 5KK1 A; 3PV0 A; 2VT2 A; 3RKY A; 2VON A; 3PQJ A; 3QPH A; 3FXR A; 3DFG A; 4BH9 A; 1Y0U A; 3LST A; 2HYF A; 2NN6 I; 2W84 A; 5DD4 A; 2E7X A; 1EH8 A; 3H94 A; 4GYG A; 4I98 B; 1Q90 A; 1D8J A; 3E3V A; 3M8E A; 5FD6 A; 2DK5 A; 3VR6 A; 3F21 A; 2P7V B; 1KQ8 A; 1GUS A; 3GFI A; 1HCZ A; 4LD5 A; 2K4J A; 1FR3 A; 1I27 A; 3ND9 A; 3CO6 C; 3I4P A; 3RPQ A; 4HF0 A; 3WOD F; 4HX4 A; 5F1R A; 3DPL C; 3E6M A; 3BG3 A; 3CRK C; 5ERI A; 3I4L A; 1U2W A; 4H0E A; 5LHI A; 4KT5 C; 3ND8 A; 2F5D A; 2CSO A; 1STZ A; 5E18 F; 1R00 A; 3DKW A; 1U5T C; 1BI2 A; 2DFI A; 1O3S A; 4P55 A; 3SXY A; 4BHC A; 3HHH A; 1J2X A; 3R6S A; 1PUE E; 2EV6 A; 4A12 A; 1TNT A; 3OOP A; 4PDK A; 2WV0 A; 1UFM A; 4HYE A; 2O8X A; 1Z7T A; 3VFZ A; 2DT5 A; 1HSJ A; 4L5I A; 4UVB A; 3KLR A; 3B02 A; 2HTJ A; 1BM9 A; 3NRV A; 1T38 A; 2HZT A; 2F5C A; 3ML6 A; 1J7K A; 2EV0 A; 1QPM A; 1TW3 A; 4N9I A; 2DN8 A; 2RN7 A; 4DQ2 A; 2Y0M A; 3DXJ F; 3VR3 A; 4MQ9 F; 1IW7 D; 3QOP A; 3KEO A; 3GXO A; 4AIH A; 2OBP A; 3W6J B; 1AOY A; 2D2W A; 3BPX A; 4AIK A; 3I58 A; 2Z3Y A; 1RI7 A; 3WGI A; 2ZNY A; 4M9Z A; 5LBQ A; 3MFY A; 1HW5 A; 2BE5 F; 1YNN C; 3I72 A; 2NS0 A; 2XAH A; 3I59 A; 2B0L A; 1HBX G; 2KKO A; 3I59 B; 4RB3 C; 2LSO A; 3IHU A; 1U5T A; 5JVT A; 1XSD A; 1U5T B; 1LEA A; 2YU3 A; 4WXD A; 2ACJ A; 1Q1H A; 3P9I A; 5ED4 A; 3GVA A; 1TTY A; 2P5V A; 4ESF A; 4I2O A; 5E1Z B; 1GXQ A; 1P92 A; 4HQM A; 1BIA A; 4A6E A; 3CDH A; 1UCR A; 4Q47 A; 2EK5 A; 3H3Z A; 1IW7 C; 2RKH A; 4PZJ A; 1OYI A; 3K1M A; 1IUY A; 2E76 C; 4EJU A; 2V1D A; 4Y13 A; 1B6A A; 3P7N A; 4WWC A; 3CUO A; 1HW2 A; 4YII A; 2W24 A; 1QJO A; 4M71 A; 2L4A A; 4EGZ A; 2IA2 A; 5CYV A; 4L0Y B; 1WWX A; 2NNY A; 2V4D A; 2F2E A; 4O6J A; 1S29 A; 4CO8 A; 3WTY C; 1YQA A; 2ZJ2 A; 3R0J A; 3V7S A; 3I53 A; 4HF1 A; 1DPU A; 3I5U A; 4Q4Z F; 1HKQ A; 2IA1 A; 2L01 A; 3RKX A; 1LB2 A; 3WU1 B; 5JFR A; 4M6Y A; 4WX9 A; 2K7L A; 1TKW B; 4M6X A; 2Y48 A; 5HS8 A; 2D1H A; 1HQM C; 1SD5 A; 3OPO A; 2QEN A; 1XB4 A; 3M7G A; 3V8J A; 5A2Q K; 3ZMS A; 2XIG A; 1OKR A; 2KJB A; 2JSC A; 4KIC A; 1FWZ A; 4ENM A; 4I09 A; 3HGB A; 1R5G A; 2XVC A; 2PQ8 A; 2K86 A; 2V9V A; 3K9T A; 3GZ5 A; 2MH2 A; 3C18 A; 4UXN A; 5K1Y A; 3EQL C; 1J59 A; 5CVJ A; 1XDS A; 3GYH X; 5DYM A; 3VOE A; 1XGN A; 3HIF A; 4LK0 F; 5C0X G; 3TO6 A; 3PUY A; 3ZQ7 A; 1TW2 A; 1H9J A; 1H9T A; 2OQG A; 2KIM A; 4LMY A; 3ZN1 A; 5H20 A; 1I6V C; 3FXQ A; 4YG2 F; 1DDN A; 2XAQ A; 2R6G A; 1FZP B; 3H92 A; 2DBB A; 3LWU A; 4DNT B; 5E8G A; 1E2Z A; 1HW1 A; 3T8R A; 3C7J A; 2VBW A; 2HR3 A; 1G3W A; 3GW2 A; 2Q8I B; 1W4M A; 3I54 A; 4H13 C; 1UHW A; 4RGX A; 2CGP A; 1DXM A; 4EM1 A; 1GHJ A; 1XSX A; 3JSP A; 1VCI A; 2DO7 A; 3WTW C; 5BN4 A; 5LLQ A; 2VE8 A; 3D4R A; 4OO1 H; 1IW7 F; 4ZYD A; 4QWQ A; 2Q2E A; 2M1B A; 2UXX A; 4IRI A; 1DUX C; 2HS5 A; 3RDI A; 1T6S A; 2YX7 A; 1W5S A; 3IC7 A; 2AUK A; 4U7B A; 3LAJ A; 4WXH A; 3E5X A; 2LDU A; 3RIR A; 4RGS A; 2QBY A; 3HSR A; 3ZMD A; 2QBY B; 2HOE A; 1IRF A; 4XLP F; 2PMU A; 2XHK A; 5FGM A; 1FNN A; 2F1M A; 1U78 A; 1TQI A; 2PBI A; 1V3F A; 3TDZ C; 3KLN A; 2ETH A; 5HDN A; 2MD5 A; 3S2W A; 2BA0 A; 3FXU A; 3D31 A; 5ICF A; 3N4M A; 1SD6 A; 2RVC A; 1RUN A; 3M85 A; 4HR7 B; 2L3D A; 5DCM A; 3EET A; 5DCM B; 5L3E A; 2V1U A; 2P4W A; 2PPB C; 1C0W A; 1J0R A; 4AWX B; 3IRR A; 4GXO A; 1Z6H A; 3FPP A; 1CI3 M; 3JW4 A; 5DUK A; 3I73 A; 1YS6 A; 3L09 A; 1G29 1; 4L18 B; 2IP2 A; 3AB9 A; 1I1G A; 3IL2 A; 2COM A; 3EQL F; 4RCN A; 5D8L B; 2P8T A; 3SZP A; 1Z7U A; 1WKM A; 1B9M A; 1OXV A; 5HVQ C; 1GJX A; 2XKP A; 1REP C; 2HQN A; 3R60 A; 4LGW A; 3OW7 A; 3HHG A; 3P9C A; 4ENN A; 1LEB A; 1MZB A; 3IKV A; 4HDU A; 2VOP A; 4S05 A; 2JTV A; 3DF8 A; 1AWC A; 2H6B A; 2IRF G; 2EVB A; 4ZYE A; 4OOI A; 1G3S A; 1H9K A; 4BHB A; 3WTS C; 3BPV A; 2MC3 A; 2XAJ A; 2XUB A; 4HV6 A; 2WWY A; 2NYX A; 1ZRD A; 1LAC A; 3FWE A; 4KMF A; 1BC8 C; 2E75 C; 1S8N A; 3RYP A; 4EJO A; 1G4D A; 1BDO A; 1TNS A; 1D8K A; 4OIN F; 2X0L A; 4IVN A; 5D5X B; 1FOK A; 3RKW A; 1Y8N B; 3QU3 A; 4R1H A; 2ZO4 A; 1IRG A; 2KJC A; 2L02 A; 2V1X A; 4O5V A; 1UB9 A; 5CVV A; 1LJ9 A; 1P4W A; 4G7O C; 4AIJ A; 1ON2 A; 1U3E M; 3U2R A; 5J8C A; 4M9X A; 1GUT A; 2CQK A; 4ZZD A; 1ZKO A; 3DEU A; 2MH9 A; 3LQQ A; 4ENK A; 2PMH A; 4OIO F; 2GXG A; 4N0B A; 2BE5 D; 1H9G A; 4RGU A; 2JXM B; 5DCF A; 2R7D A; 5ILV A; 1R1U A; 4I7Z C; 4KDP A; 3DP7 A; 3B73 A; 4UVA A; 1XGM A; 1B1B A; 5L3D A; 1FYC A; 3KZY A; 5K5O A; 3VEP A; 2A3S A; 1ON1 A; 2RNJ A; 1QQI A; 4DNR B; 5E17 F; 4KKS A; 3ELK A; 2FNA A; 4LLG F; 4XRF A; 4JK1 X; 3FMQ A; 3HOS A; 4HX8 A; 2O03 A; 2HEO A; 3MWM A; 1Z6T A; 2HWV A; 2L5T A; 3EUH A; 5D5V B; 1ZG1 A; 3TDU C; 1BBY A; 4L5J A; 1ZG5 A; 2G7U A; 1L3L A; 3I0T A; 4PV1 C; 2AUJ D; 3ZMZ A; 3DPT A; 2FMY A; 2IVM A; 1MJA A; 2ZXJ A; 5K36 H; 2VBZ A; 2WTE A; 3GFL A; 5CJ9 A; 2O5J C; 2FRH A; 1K6O A; 4U0V A; 1BC7 C; 2A6E D; 1RUO A; 1QZY A; 2TDX A; 2EFP A; 4KMU C; 4KIF A; 3GO5 A; 3HSE A; 4TV7 A; 3W6K B; 3BJ6 A; 1X3U A; 2GA1 A; 1GUG A; 1R1T A; 5C0X H; 1FSH A; 4E70 A; 5CVU A; 1GHC A; 4D0P A; 1ZLJ A; 3J81 K; 4GO1 A; 5KND A; 1B59 A; 2KCC A; 4G6D A; 1J75 A; 3KP4 A; 1LQ1 A; 5AON A; 3MAJ A; 4MTD A; 5LGT A; 2O6G E; 4B09 A; 4L9N A; 3HSF A; 2EDG A; 5TJJ A; 3NQO A; 4HX7 A; 2FE3 A; 2UZK A; 3P20 A; 2A68 D; 1XN7 A; 2JT1 A; 3AAF A; 5IT9 Z; 2A6E C; 4P5O A; 3O6B B; 2R7F A; 3BOQ A; 1Z1D A; 1GXP A; 3KCC A; 4HV5 A; 3QJY A; 1I1S A; 3TO9 A; 1R1V A; 4G9Y A; 3PUV A; 2ESH A; 1W5T A; 4A5M A; 2HTS A; 1FSE A; 2F5E A; 1K78 A; 3M8F A; 4G7O F; 4P9F A; 1K78 B; 2Y75 A; 2PG4 A; 2UXN A; 3IRQ A; 2DPD A; 3V7C A; 2WC2 A; 3KEQ A; 4HA8 A; 4CYD A; 3VOD A; 1F5T A; 2YYZ A; 3K2Z A; 1NHA A; 2A68 C; 1BN5 A; 3ULQ B; 1OYY A; 5L3G A; 1LNW A; 2O0Y A; 2KIF A; 1G3T A; 3SZT A; 1Q1B A; 2QUF A; 3L9F A; 3E6C C; 3FF5 A; 2OD5 A; 2OU2 A; 3U9G A; 1KQ9 A; 2W85 A; 1IN4 A; 3C3W A; 1SFX A; 3FMR A; 1GUO A; 2FBK A; 1K8O A; 4H0L C; 1FBQ A; 1BIB A; 1ZAO A; 3RPU A; 5D5W B; 3GF2 A; 3JTH A; 3IWF A; 1S3J A; 1YYV A; 3RTR A; 5FFZ A; 3SFZ A; 4CDG A; 4EM0 B; 1A04 A; 2ZFW A; 1PP7 U; 2E1C A; 3NE5 B; 1JXS A; 2CYY A; 4F7Z A; 3QRF F; 3PUX A; 4XTC S; 4Y15 A; 4L9J A; 2GZW A; 3MZH A; 3LA7 A; 2PEX A; 4BAY A; 1T2K A; 2CG4 A; 3QU6 A; 1WRJ A; 4BHP A; 5ILU A; 2EJM A; 1A6X A; 1ONL A; 3JTG A; 4CGZ A; 1KQ0 A; 4HDV A; 2J5O A; 1SAX A; 5LGU A; 4EM2 A; 4XSZ F; 5HOO A; 2RV8 A; 3VB2 A; 2XV4 S; 4ENJ A; 1XDU A; 3KE2 A; 2XAF A; 2A61 A; 1XGS A; 4ESB A; 3E5Q A; 4FT8 A; 2DK8 A; 4BQA A; 1FX7 A; 2QYO A; 2PN6 A; 2GXB A; 3A8J E; 1XSV A; 1GVJ A; 1T5E A; 3W3A A; 3IFT A; 2XRO A; 4WCG A; 4U0Y A; 3EYI A; 2A6E F; 3O2P E; 1O3Q A; 1OMI A; 2DI3 A; 4KHZ A; 4CXF A; 4KI0 A; 4YFX F; 1YFH A; 4A2U A; 2STW A; 3MCZ A; 3F8F A; 2FML A; 4UV9 A; 2CWE A; 3WE2 A; 4TKO B; 3QAH A; 3QIA A; 3COA C; 1R7J A; 2LKP A; 2ZKZ A; 3SQN A; 3WGH A; 2QWW A; 3O3K A; 2PCF B; 5KNC A; 2IT1 A; 4HBL A; 5EEH A; 3GD7 A; 2OZ6 A; 3WDN A; 3T51 B; 1T0F A; 2A68 F; 2EA4 A; 1J9I A; 3R61 A; 5AIQ A; 4P96 A; 4HZF A; 1B4A A; 4HLX A; 2R3S A; 3RI4 A; 5HDG A; 4EMS A; 2B8F A; 1ZH5 A; 2ZNZ A; 3LFK A; 2ZBK A; 4PGG A; 4IPA A; 3BJA A; 2EJR A; 3WTX C; 2K32 A; 3FZV A; 1FP2 A; 5LEK A; 4IFD G; 3HUG A; 3K1N A; 3M1E A; 4Q5S F; 2ESN A; 1SD7 A; 3T0Y A; 1PP8 F; 4XIG S; 1UST A; 3OOC A; 3HRS A; 1VTN C; 3KFW X; 5E1W A; 2FA5 A; 1FY7 A; 2VBX A; 4ZYG A; 2IJL A; 3K0L A; 5J8F A; 4RAZ A; 2E1N A; 2LMC B; 1V8Q A; 2JRT A; 2HFH A; 3N97 A; 1K8M A; 4OIP F; 3CUQ C; 4Q48 A; 1KU2 A; 3BZ6 A; 2BDO A; 3F22 A; 1G6N A; 2HKO A; 1EV7 A; 1QZZ A; 5E20 A; 1ZGJ A; 3J80 Z; 1F9N A; 3H3U A; 1H9S A; 4ZYH A; 2M45 A; 1H9S B; 4Z2Y A; 5L3F A; 4JQF A; 1YG2 A; 1H9R A; 3FX3 A; 3E97 A; 3DV8 A; 4HQE A; 3TZU A; 5D4R A; 1SMT A; 3T1B A; 2W25 A; 2XRN A; 3GEZ A; 3TO7 A; 2EJG C; 2PFB A; 3N6R A; 4KIB A; 3L7W A; 2D5D A; 2QZ8 A; 1K79 A; 4LFU A; 1Q1E A; 3F23 A; 1D5V A; 3A7L A; 5AIP A; 2K02 A; 5HLI A; 4WXC A; 3GFM A; 4EJV A; 5LEJ A; 2OZU A; 3J7A O; 4GCV A; 2A5Y B; 5BPD A; 2DU9 A; 3ZN0 A; 3F8M A; 5H6R A; 2DNC A; 3MFK A; 4KN7 X; 2XAG A; 1EH7 A; 5J6X A; 3HRU A; 1FPQ A; 2DQR A; 4IHS A; 3F3X A; 5H6Q A; 3HTS B; 3A7A B; 3RLF A; 3TOB A; 3WE3 A; 1OXX K; 2FQ3 A; 4RB1 A; 2IT0 A; 4TQU S; 4L9V A; 3M4Y A; 2M5W A; 5JHU A; 3PUZ A; 2YX4 A; 1GUN A; 1Q12 A; 1DCZ A; 1MGT A; 3MXU A; 2MLK A; 5FFX A; 2EA2 A; 2KRF A; 3QPT A; 1BI3 A; 1H0M A; 2OQR A; 1V43 A; 2QVO A; 2ELH A; 2GA2 A; 2MLG A; 1IF1 A; 4KN4 X; 1XD7 A; 1YW8 A; 3R0A A; 3L00 A; 2VN2 A; 3MEX A; 2M30 A; 3DQV C; 4UVC A; 4IXA A; 2GWR A; 3KP6 A; 4L0Z B; 3VA7 A; 3EYY A; 3WTZ A; 5EEG A; 3BDO A; 2VC0 A; 1JHH A; 1USS A; 1YLF A; 2RDP A; 2P5L C; 4OGQ C; 3T8T A; 4Y17 A; 4LG0 A; 5BN3 A; 5CLS A; #chains in the Genus database with same CATH topology 4U88 A; 2MYS A; 4ZKB B; 1TU2 B; 2I5L X; 4A3M H; 2CV2 A; 1OXT A; 1K4S A; 4X3U A; 1GLN A; 3S16 B; 5JEA G; 3L1P A; 4C1N I; 4FXD A; 1DD2 A; 1N36 L; 1WTQ A; 1Z47 A; 4CIC A; 1FD7 D; 2NN6 G; 4FQG A; 1B53 A; 3PSF A; 4JK1 C; 2DA2 A; 2L54 A; 1PRT D; 2E1M C; 3HDD A; 3HRM A; 4IZZ A; 2HDW A; 1GXD C; 4ULL A; 2PMZ A; 2MC4 A; 1YNJ D; 2I8D A; 1AU7 A; 1X4P A; 3AG3 H; 1OFC X; 3KYH C; 3KYL A; 2DQL A; 3HG9 A; 5DM7 F; 3HKZ B; 3GTO H; 2LFN A; 3CCM A; 2L4M A; 2V1C C; 2ZXH A; 3H35 A; 4C3J G; 1MMN A; 3S24 A; 3IWZ A; 3GTG J; 4UNO A; 5FLX Z; 4OIR F; 5H1S E; 1P4X A; 3HOT A; 1EY7 A; 4R9W A; 2RC4 A; 2A6H D; 2M34 A; 3LFU A; 2O8K A; 4CSW A; 5JJK A; 5FLX L; 2D1V A; 1WTO A; 3QP5 A; 3ASO H; 2Z1D A; 1VQK I; 5JPN C; 2XRL A; 1Q1V A; 3VDX A; 2PI2 E; 4X65 L; 2JA2 A; 5DDG A; 4RAF A; 1ETO A; 4PJM A; 1ZRF A; 2GLO A; 3HOY H; 4XLF A; 4KNY A; 1ZYR C; 3VR2 A; 1L1O A; 4XON A; 1YTY A; 4E7H A; 3RUZ A; 1ZHF A; 3I56 I; 4BBS J; 4FHT A; 1JC7 A; 2NVY H; 1I94 L; 5IGB A; 4DR2 Q; 1ZQ3 P; 2IW5 A; 3JAM Z; 4P75 A; 3DPU A; 5BMZ A; 4LJZ C; 1TC3 C; 3H3V I; 2CW0 C; 1O7F A; 4AYB E; 5EPL A; 3I55 I; 3MA2 B; 4PNY A; 4JI0 L; 3PF4 A; 2HD3 A; 4MTK A; 2BOS A; 1T56 A; 2HDD A; 3HL2 A; 2R7Z B; 3JAM L; 3TSS A; 1N8R C; 4XNF A; 4FCY A; 1YJN A; 3S1N J; 3L7Z C; 1BR4 A; 2EB7 A; 3AKZ A; 2VJI A; 1X3E A; 4N9H A; 1K8G A; 1UE6 A; 3OMY A; 2KCM A; 1TT2 A; 1IZ6 A; 3KJO A; 4P9U A; 4UUV A; 4CCO A; 2QCP X; 5D5U B; 2XDV A; 1QWY A; 2K28 A; 1STH A; 2BY3 A; 1S7A A; 2BGC A; 1R58 A; 4ZTF A; 1L0O C; 3BTL A; 1WJ5 A; 2XPW A; 3HB9 A; 1MND A; 4X0G A; 1RF2 D; 2K27 A; 4LOC A; 1EH6 A; 2KBN A; 2STT A; 3M4O J; 3V4G A; 3IAM 2; 2AQ2 B; 1VMP A; 3ZMV A; 2EFQ A; 3GZ6 A; 1EU4 A; 1X19 A; 3J7Y J; 3OYG A; 4XQF A; 4GLX A; 1SMY F; 2EYO A; 4XIC A; 3E9H A; 4BE1 A; 1MSH A; 2VJ5 A; 3HXL A; 1MNE A; 1VQK A; 3K59 A; 3CCY A; 1HNZ Q; 4LFB Q; 1BOS A; 3WG9 A; 1UHM A; 2FNP A; 1KON A; 3K6O A; 3DMU A; 5E3N A; 2X6X A; 3I56 A; 4WK8 F; 3BR6 A; 3HOV J; 2O9L A; 1ZN2 A; 5GAH J; 1RZ4 A; 2E2D C; 3J81 j; 1Z05 A; 1BJA A; 3MN2 A; 4DR5 L; 2F5F A; 3KET A; 2K52 A; 4UB8 C; 3LOC A; 1NJI C; 1IBK Q; 4HPQ A; 4BY1 B; 4BEH A; 5LD2 C; 4BXF A; 5FB2 A; 2OQ0 A; 5CBE E; 1E2X A; 1O3R A; 3BYY B; 4RB2 C; 2V6C A; 2R92 H; 4F93 B; 2NTS A; 1R49 A; 5IGE A; 3NAU A; 3S3O A; 1XTC D; 3S32 A; 5ELC A; 2X69 A; 3DWA A; 2UUC Q; 4BE0 A; 3P1H A; 3FXL A; 3CD6 I; 4G94 A; 5B1A H; 4PK4 A; 3ISP A; 2IS6 A; 5EEA A; 5FJ8 J; 1R23 A; 3T5X A; 4UV8 A; 5DTD A; 1MBY A; 3BRO A; 1EIF A; 3F6O A; 4UUT A; 4EAT A; 5HLT A; 2X1D A; 3FXJ A; 1X1C A; 4AD9 A; 3FIS A; 4G8X A; 3T53 B; 2MFI A; 1LTL A; 1JE8 A; 4XKW A; 4R4C A; 4UP7 A; 3S1N B; 1QG7 A; 4LK1 F; 3OYL A; 1UE1 A; 1ENH A; 1GV2 A; 2H8W A; 2OA4 A; 3MXN A; 1KGS A; 1H9M A; 2CUF A; 5D6F A; 2PJR A; 4J00 A; 2XAS A; 5GAH C; 3D3R A; 1LTI D; 2VQG A; 1DP3 A; 3X3N A; 4I99 C; 1N25 A; 1BVS A; 4LF7 Q; 3C2G A; 1VQ4 I; 5HUR A; 3S1M H; 2DMU A; 1U4L A; 4O1N A; 4NKL A; 2ID3 A; 1BI0 A; 3GLL A; 2HHH L; 2WV1 A; 1GUW A; 1LVA A; 3J80 i; 3QG1 A; 3JRH A; 1O57 A; 4IHT A; 4CHT B; 3Q8D A; 1W7P D; 4G7H C; 4IFD I; 5J22 A; 1IXC A; 4G12 A; 4NC6 A; 3MMY B; 3HOZ H; 1XGO A; 2VUM G; 3TP5 A; 4DJA A; 4YFN F; 2BV6 A; 1OLT A; 1L0W A; 2E5L Q; 1TLH B; 1FYL A; 2QQ8 A; 3NHH A; 4UUS A; 5IT9 K; 3HKZ E; 5ELN A; 4EQN A; 4KN7 C; 5JPM C; 2OAZ A; 1U4M A; 2A69 C; 3CUQ A; 1MJC A; 3FHZ A; 3HOV B; 1WTP A; 4IQZ A; 4A3D J; 2EQS A; 5CVI A; 5L37 A; 2X6Y A; 4A93 H; 5LGN A; 1B72 A; 2Y69 H; 2F4M B; 3FHN A; 2BXY A; 4LB5 A; 4DV5 Q; 3LWF A; 1P6R A; 5DV5 A; 3LSM A; 1N36 Q; 3ESW B; 4RS8 A; 1X55 A; 3S3N A; 3A8K E; 3H0G H; 4PMB A; 1M8A A; 5ICE A; 1ULY A; 2RQQ A; 2AHO B; 1CA5 A; 5F6F A; 2HNH A; 4M74 A; 3CLQ A; 3BZK A; 5EG0 B; 3VSL A; 1VDZ A; 1HA5 A; 1MD0 A; 1CSP A; 3E9I A; 4P73 A; 2XGX A; 2FIN B; 1FBS A; 4A0X A; 4G7Z F; 1HJP A; 3OWE B; 5ELO A; 2L7K A; 2LBF A; 1EYD A; 3IH3 A; 2ZME C; 1VQ4 A; 5F64 A; 1YX3 A; 5C4Z A; 5ISR A; 3FDY A; 1FC3 A; 1ENA A; 3C3L A; 3F72 A; 1YEZ A; 5A2Q E; 1KAB A; 1FLI A; 3BR3 A; 4IOA F; 3BU2 A; 1FYK A; 2CKZ B; 2LSS A; 1XYI A; 2FBI A; 3GTQ H; 4F88 A; 1K9M C; 4E7S A; 1O80 A; 3G2B A; 4JBM A; 3D4W A; 4YIF A; 1EFW A; 2P6S A; 3EFX D; 1AP0 A; 2FHT A; 1E0B A; 4A3E J; 4X65 Q; 4LF4 L; 1Y1V H; 2GIV A; 3PIP F; 4NUB A; 3RNU A; 5BN5 A; 4M3G A; 4YWM A; 6PAX A; 1PH2 A; 4NQW A; 2L11 A; 3LA3 A; 2VPR A; 1R5H A; 4NNJ A; 1IV6 A; 4A3K G; 1I94 Q; 5ICG A; 1IN8 A; 1HUN A; 2Y31 A; 2P7C B; 4H44 C; 1CQV A; 3SJM A; 1TS3 A; 3S16 H; 2ZME B; 2K9N A; 2G7H A; 4A3B B; 5BS6 A; 5KK1 A; 3L2U A; 3GBG A; 2AQ3 B; 3A02 A; 4NXN L; 2LKX A; 1N34 L; 4JI0 Q; 4BXX G; 2KIQ A; 1QRY A; 3DFG A; 1A35 A; 2HYF A; 5HNF A; 2JK8 A; 2HYJ A; 1RIP A; 4GYG A; 1JUM A; 4XLA A; 4KJZ A; 1JKQ C; 3VR6 A; 1W36 C; 4BJ5 C; 4XLH A; 2K4J A; 1KAW A; 4NP5 A; 4HF0 A; 3WOD F; 3S93 A; 3DPL C; 3E6M A; 3BG3 A; 2ASB A; 4H0E A; 4DV0 L; 1I50 J; 2EIL H; 2AKW B; 1VQ7 A; 3PNT A; 2ID6 A; 1VQ6 I; 1QE6 A; 1U5T C; 5GTH C; 2DA4 A; 1O3S A; 2Z4I A; 4BHC A; 1PRT B; 3H5N A; 4CDC C; 1PUE E; 4Q5V A; 1TNT A; 4PDK A; 1UFM A; 4QUC A; 2O8X A; 3ZCO A; 2DT5 A; 1YNN D; 4OO1 I; 1WTH A; 4L5I A; 4UVB A; 1R5I D; 3B02 A; 3KLR A; 1T38 A; 1Q81 C; 1IKM A; 4ME5 A; 5C4X G; 1SFO J; 1LT5 D; 4ZAI A; 3QQA A; 2EV0 A; 2OV7 A; 1D5Y A; 2F4I A; 3OYB A; 3KJP A; 1I6H J; 4B3R L; 1LTR D; 4UPA A; 1TT0 A; 2M4G A; 3BQZ A; 4P72 A; 3G4S I; 3A56 A; 3I58 A; 1NG7 A; 1YIT I; 3CQZ B; 2ZNY A; 2VQF L; 4A3G J; 3ONQ A; 5LBQ A; 4II3 A; 4DR5 Q; 3I72 A; 4HED A; 2LY9 A; 3OS2 A; 2RDF A; 2RSO A; 5JVT A; 1XSD A; 1U5T B; 1JOK A; 4DV7 L; 4O42 A; 1MMS A; 3JRI A; 2R7Z H; 4F91 B; 2F0L A; 3P9I A; 1VQN I; 2DMT A; 1Q46 A; 4I2O A; 3ZQF A; 5E1Z B; 3ZGK A; 1GXQ A; 4HQM A; 3V05 A; 1BIA A; 2B2W C; 4DNI A; 4Q47 A; 1ZGW A; 2X9D A; 1MGS A; 4PZJ A; 4XKB A; 1OYI A; 1IUY A; 1ILP A; 4B1R A; 1YNX A; 3AMU A; 4BY7 H; 2XF5 A; 4YII A; 1QJO A; 5M9O A; 4EGZ A; 4IPG A; 4JG2 A; 1WWX A; 1I39 A; 4N8X 1; 2EIF A; 2F2E A; 1S40 A; 4XXE A; 2HVQ A; 1S29 A; 2HDL A; 4CO8 A; 1NTG A; 2DXI A; 3KFU A; 4UP8 A; 3I5U A; 1HKQ A; 4LG3 A; 1NNX A; 4RHS A; 3IAY A; 2Z1C A; 4YCW A; 3EUK L; 1SBB B; 3FVQ A; 4IO9 A; 3M7G A; 5A2Q K; 2WAQ G; 4DN4 M; 3P5J A; 4YCV A; 1FWZ A; 5C3E J; 5J40 A; 3H91 A; 4PZ6 A; 2F03 A; 3X3M A; 2OTL I; 1P16 A; 3K9T A; 5C44 G; 5E1F A; 3PO3 B; 5F1J A; 3GYH X; 3J81 i; 1XGN A; 1MBK A; 3J7A Y; 3GTG H; 2YU9 J; 5I6Y A; 1STA A; 4DV6 L; 4DF7 A; 1M90 C; 1S7E A; 2KIM A; 2M2E A; 4JI2 L; 5EKK A; 2HHH Q; 1I6V C; 1TWC J; 2WAQ N; 2XAQ A; 3ZJY C; 2DMN A; 3BT9 A; 1EA3 A; 3NBH B; 2CQ7 A; 4X3S A; 3BG5 A; 4HIO A; 1TT5 B; 3S2D J; 4H13 C; 5BNG A; 1WCM B; 1H89 C; 2K05 A; 3HC7 A; 1XSX A; 1VCI A; 4GLA C; 2TCT A; 2XF6 A; 3DM3 A; 4BBS H; 2LS0 1; 3MC0 B; 5BN4 A; 4IFD J; 1IW7 F; 1K6Y A; 4QWQ A; 1WFQ A; 2M1B A; 4IRI A; 2Q2E B; 4B9X A; 3OY9 A; 3TP0 A; 4BAC A; 2MGS A; 4AC0 A; 1PH8 A; 5FLX c; 3IC7 A; 1Z0X A; 2COB A; 4WXH A; 4B3X A; 3RIR A; 3HSR A; 2QBY B; 1CUK A; 3A01 B; 1OCT C; 2PBI A; 4NC7 A; 4XM3 A; 5LL6 X; 1V3F A; 4I65 A; 2WAF A; 5IYD J; 2LKV A; 2BA0 A; 3FXU A; 2EPB A; 3D31 A; 5ICF A; 3N4M A; 4DFA A; 1SD6 A; 1U9N A; 3NEL A; 2ES2 A; 2RVC A; 1YX5 A; 3H9G A; 4HR7 B; 5BR8 L; 2L3D A; 1VND A; 3EET A; 5AFW A; 1QNK A; 1C0W A; 1YIJ I; 1EWC A; 3IRR A; 1SYB A; 1CI3 M; 3MWY W; 3A4C A; 2FMC A; 1BR9 A; 3AB9 A; 3S1R J; 2HQ5 A; 4Z9C B; 1I1G A; 3PF5 A; 2GA4 B; 1WTW A; 3ZQQ A; 1WKM A; 2WFW A; 3G7L A; 1B9M A; 5HVQ C; 3M4O H; 4IOA A; 1RKW A; 1REP C; 3MD2 A; 3P9C A; 4HDU A; 4S05 A; 3CP0 A; 4XID A; 3BC8 A; 3U4V A; 4F52 A; 4ZYE A; 5C3E B; 1II3 A; 3WTS C; 1K3R A; 1LAC A; 2VB3 X; 4Y52 H; 3CPF A; 3FWE A; 1OTC A; 4KMF A; 1BC8 C; 2MNA A; 1MBE A; 3PIP A; 1HUM A; 4KJN A; 1RJT A; 2N55 A; 2WAQ A; 3W2V A; 3AFP A; 3RKW A; 1Y8R B; 3QU3 A; 4QF4 A; 2YU9 B; 1IRG A; 1UUP A; 4LF4 Q; 3CCR A; 4O5V A; 3HOV H; 3VYS A; 1UB9 A; 4A3F B; 5C5L A; 4G7O C; 1ON2 A; 4QUF A; 4A76 A; 3CMA I; 2KLO A; 3U2R A; 1QZH A; 1GUT A; 2CQK A; 1DGS A; 1ZKO A; 2FMM A; 3PGZ A; 3CC4 I; 3LQQ A; 3RMH A; 2PMH A; 3S1Q B; 1H9G A; 3DUZ A; 3E0J B; 2LD5 A; 1R1U A; 3KF6 A; 3SZG A; 5G3L D; 1XGM A; 1B1B A; 1QHG A; 3KZY A; 4NXN Q; 5K5O A; 2CQQ A; 3VEP A; 1WJE A; 1N34 Q; 4GNX C; 1V1Q A; 3ER0 A; 2RNJ A; 1WVL A; 3ELK A; 2FNA A; 4GOP B; 2L7M P; 4JK1 X; 3HOU J; 1WHU A; 1QVT A; 2O03 A; 2JN4 A; 2HEO A; 4OHJ A; 3GTP J; 1JT8 A; 2E5Z A; 3TDU C; 2PZW A; 4L5J A; 1L3L A; 3TQY A; 1AE3 A; 2VQC A; 2R7U A; 2IVM A; 1A15 A; 1PV4 A; 2JQ7 A; 1FJG L; 5K36 H; 2CUJ A; 1QVU A; 4GN3 B; 1LTS D; 3K4C A; 4DZP A; 3BDC A; 4DV0 Q; 1JTX A; 2CWP A; 2A6E D; 3KLS X; 2UUA L; 4ALP A; 4DR4 L; 2EFP A; 5IPA A; 5CPR B; 2EQR A; 3GO5 A; 3HSE A; 2ME0 A; 1FIP A; 1X3U A; 4JYK A; 1FSH A; 2HQA A; 3SEQ A; 4D0P A; 2R3Z A; 3J81 K; 5KND A; 2KCC A; 1EWI A; 2LR8 A; 1J75 A; 4AIM A; 1W0U A; 1IBL Q; 3CCQ A; 4MTD A; 2O6G E; 4L9N A; 3LX0 A; 4RMF A; 2BVM A; 3FPU B; 4KIT B; 1N32 L; 2K9S A; 3CC7 A; 2DMP A; 4JP0 A; 1IXR A; 3U21 A; 3S15 H; 3S1R B; 3CCE I; 1BO0 A; 3ZR8 X; 4P5O A; 2R7F A; 2X1C A; 5HY6 A; 4G9Y A; 3PUV A; 1DOL A; 3RZD J; 4B3R Q; 3CMA A; 2HTS A; 1K78 A; 3M8F A; 2Y75 A; 3IRQ A; 2VQF Q; 4C3H G; 3BLY A; 1D5X C; 2PZT A; 2CJJ A; 1J9O A; 1ZK8 A; 4M3E A; 4CYD A; 3VOD A; 2DT6 A; 4C3I J; 1H8A C; 3FHW A; 2YYZ A; 2EYJ A; 4DV7 Q; 2A68 C; 4DOZ A; 3I4M G; 3CP8 A; 1OYY A; 1SNQ A; 4GN5 A; 1LNW A; 4H02 A; 4IZ8 A; 1WPK A; 3F1Z A; 3FXK A; 2OD5 A; 1KQ9 A; 2W85 A; 2XRQ D; 3C3W A; 4A3D H; 3SDB A; 2I5M X; 1K8O A; 3RPU A; 1VQJ A; 1EY9 A; 3AG4 H; 3GTJ B; 2IL8 A; 2DOF A; 1YYV A; 1SND A; 3NE5 B; 3RKQ A; 1JXS A; 1CKN A; 2SEB D; 1JJ2 A; 3IFD A; 3PUX A; 4XTC S; 4Y15 A; 4DYR A; 4L9J A; 2GZW A; 4ZDL A; 3LA7 A; 4BAY A; 2N8N A; 2CG4 A; 2N88 A; 3PM1 A; 3VYU B; 1A6X A; 1VQG A; 5T1I A; 3BR0 A; 3VYR B; 4A3M G; 2GVA A; 1NSN S; 3DCF A; 1HR0 W; 1KQ0 A; 1SAX A; 1F9R A; 1ZR2 A; 4EM2 A; 1F36 A; 4A3J J; 1DGR M; 3VB2 A; 3RR5 A; 2XV4 S; 1XDU A; 3MEH A; 2E34 A; 1VQB A; 1EEF D; 1XGS A; 3E5Q A; 1LLR D; 2CWA A; 3A8J E; 3CCE A; 1GVJ A; 3CZ6 A; 3IFT A; 3OYN A; 5B66 C; 4V2G A; 4V19 0; 1Y56 A; 3O2P E; 1O3Q A; 2XSJ C; 4LUZ A; 4DV6 Q; 4CXF A; 1YFH A; 1ZTR A; 3NAR A; 4A2U A; 1GVP A; 5GP9 A; 4S3S A; 4JI2 Q; 2K85 A; 4DR3 L; 1A6Q A; 1A8V A; 2PCF B; 3OYH A; 3L2W A; 4YEL A; 2IT1 A; 4IPD A; 2XN9 C; 3KUP A; 4A3E H; 5IYC G; 1T0F A; 1YOV B; 2EA4 A; 3J7Z I; 3V2T A; 2HI3 A; 4P96 A; 4XQQ A; 3N52 A; 1RR8 C; 1MHW C; 3PO2 B; 4HLX A; 1A5J A; 3RI4 A; 3F2B A; 4QIW B; 2K03 A; 4OAY A; 1DOM A; 4EMS A; 1STE A; 1ZH5 A; 2E8G A; 2ZNZ A; 1P7I A; 3LFK A; 4H7B A; 1KIX A; 3FKI J; 1EUJ A; 2N8G A; 3WTX C; 1HFG A; 3HOY G; 2V9K A; 3FZV A; 1JOQ A; 1HDD C; 1SD7 A; 3HUG B; 4C3I B; 1MH4 A; 4HG2 A; 2LY7 A; 3TED A; 2E2J J; 1VQP I; 5JVG F; 1VTN C; 4F8M A; 5M5Y B; 3E1S A; 1NR4 A; 2K5V A; 3H3V H; 2L9V A; 3SVM A; 3G1L A; 3CDI A; 3HEF A; 1CSQ A; 2E1N A; 1V8Q A; 1X1P A; 1AKH B; 4OAZ A; 5BR8 Q; 4RGT A; 1KU2 A; 2F0O A; 1E1O A; 1C9O A; 2FOW A; 1QB5 D; 1I50 H; 5E20 A; 4JI6 L; 1SJE D; 1ZGJ A; 3H3U A; 2RKS A; 1H9S B; 5L3F A; 1H9R A; 3LSR A; 1YG2 A; 2L7F P; 4I7A A; 5E4W C; 1F77 A; 1KQS A; 3I4O A; 3GEZ A; 5ELB A; 1MNM C; 1K79 A; 1J5E L; 1VQ9 A; 1GVD A; 1UEA B; 3A7L A; 3KDF B; 2Q8T A; 1L0X B; 1SFO H; 5M3M B; 3J7A O; 4A3J B; 1S5F D; 2IBD A; 2DU9 A; 2DNC A; 1SVM A; 4KN7 X; 2XAG A; 1OCO H; 1EH7 A; 1I6H H; 2DQR A; 4YY3 Q; 2HVR A; 4IHX A; 4KIS A; 2Z16 A; 1QUQ A; 1T9H A; 4A3G H; 3M4Y A; 1PUF A; 2KEN A; 5IE8 A; 3A5Z B; 2BH8 A; 4CKE A; 3S8P A; 3PUZ A; 2YX4 A; 1OCC H; 1EQR A; 2EXD A; 4GFB A; 1BI3 A; 1V43 A; 2QVO A; 1ICF B; 1XD7 A; 3OL3 A; 2F0J A; 4M3D A; 4U68 A; 4BBR G; 3DWL E; 3MEX A; 2M30 A; 1ITY A; 1BR1 A; 3EYY A; 2R93 J; 2YUS A; 4FO2 A; 1K4T A; 3DHQ A; 4A3I B; 4FE4 A; 1N9W A; 2R7V A; 3T8T A; 5BN3 A; 1B50 A; 1KU7 A; 5HLH A; 4R79 A; 5HGQ A; 1CTM A; 2LNB A; 4ITV A; 3I3C A; 5COY A; 1G6P A; 1VF5 C; 2IPK D; 1ETV A; 3H9J A; 2R92 G; 1LDJ A; 2XIW A; 2KPM A; 2EOT A; 5FO8 B; 3IAS 2; 4ZRZ A; 1FJG Q; 3BDL A; 3SK6 A; 1S5L C; 3JCU C; 1FL0 A; 2K14 A; 3FK7 A; 4MQV A; 5I6W A; 1ICW A; 2IS1 A; 1V55 H; 2EH3 A; 2UUA Q; 3GLX A; 1ZRC A; 4DR4 Q; 2A1A A; 4E7L A; 3GCM A; 1SMY D; 1LTT D; 1E3O C; 5C3E H; 2G9W A; 3CME I; 2NVT J; 2ISZ A; 1CFM A; 4OEM B; 2PMZ B; 1JR0 D; 4DYC A; 4EGY A; 3TW6 A; 3JR9 A; 4N8F A; 1BCP F; 4NHJ A; 3S17 B; 1O3T A; 1N32 Q; 1PVE A; 3PNV A; 1APL C; 2L15 A; 1R71 A; 3SK8 A; 1Y8O B; 3GUD A; 2BHY A; 2DOA A; 4C56 C; 2DPU A; 1WH7 A; 1B2T A; 1TWC H; 4RWS C; 2L9H A; 5CV9 A; 3L0O A; 4O3M A; 3KOJ A; 5I2Y A; 4QPQ A; 2OTJ I; 4HF2 A; 3NXW A; 1LYL A; 4PGH A; 3S2D H; 2O3F A; 1X1A A; 3NEU A; 5KCM A; 2HYG D; 4A0Y A; 2KZV A; 4HIK A; 4RLQ A; 1LUJ B; 2KQR A; 3VWB A; 4CDF C; 1I4P A; 4BQQ A; 3CF2 A; 1J5Y A; 2FQ4 A; 1SAP A; 4IFD H; 1L1O B; 3GTL J; 2EZV A; 4XLC A; 1SNP A; 4A75 A; 2O61 A; 4IEJ A; 3PFI A; 3VR5 A; 2GVB A; 2NS8 A; 3PL8 A; 3D0S A; 1Y6I A; 1QO0 D; 2JVH A; 3HOZ G; 5E3M A; 1PH7 A; 2VQE Q; 3MZ8 A; 2Z99 A; 4DW6 A; 1EQV A; 4CKC A; 4ZZL A; 5HS7 A; 4RMN A; 2F0K A; 5IYD H; 4XYQ A; 3G73 A; 2KIS A; 5IOD A; 2LC2 A; 1D0Z A; 2WY8 Q; 2JA6 J; 2R93 B; 4A93 G; 3O8G A; 3CES A; 1W1W E; 1HNW L; 2VT3 A; 3CME A; 3B6A A; 3W1B A; 4QIW E; 2Z4H A; 4MEX F; 1PVO A; 3S1R H; 1IN5 A; 2DMQ A; 2I5U A; 2RCN A; 2KCN A; 3TMO A; 4BXU A; 4ABZ A; 2LXK A; 2JMW A; 3H0G G; 3RD4 A; 4KK7 A; 3ECO A; 3URY A; 5IGF A; 3WTV C; 5CC4 A; 2B8G A; 3JC6 6; 3ZMU A; 1R36 A; 3RN5 A; 1VQI A; 3SE0 A; 4B3M Q; 5IYB G; 2KUM A; 2PI2 A; 2Z6K A; 2K33 A; 3V8K A; 4DR3 Q; 2HQR A; 2PJP A; 5LRS A; 3D0F A; 2M4K A; 2FOK A; 1G6Z A; 5ELE A; 3J7A 5; 4OL7 A; 4IX7 A; 1SFE A; 4B3T L; 5JLX A; 3IV5 A; 1ZYR F; 1X9N A; 4ABN A; 2BY0 A; 1FBU A; 5CQG A; 2F52 A; 1WD1 A; 2DJN A; 3X2Q H; 2K75 A; 1BUV T; 5IOY A; 4WF2 A; 4IJA A; 4ESJ A; 2F0P A; 2KRH A; 4C3J B; 4V2F A; 4BEH B; 4LJZ F; 1M4V A; 2CW0 F; 2XGT A; 3RZO J; 3K10 A; 4K2E A; 2PI0 A; 1XCV A; 3V42 A; 5ICC A; 2TSS A; 1SYG A; 1EOT A; 3J7A V; 3HOU H; 4QQB X; 3CJN A; 1WD0 A; 3F8C A; 4FX0 A; 3GTL B; 3GTP H; 1YW7 A; 3E0E A; 4A3F G; 1Y1W G; 1TWA J; 1ENC A; 1G51 A; 3BYT B; 4CGY B; 2B3G A; 1PDN C; 5D4S A; 4HRI A; 3I64 A; 1HPC A; 2XGE A; 3OIP A; 1HQC A; 3A0H C; 3KP5 A; 5FRN A; 2H09 A; 3DWQ A; 4AM3 A; 4IJH A; 3M9A A; 2DFF A; 4JI6 Q; 3ROU A; 5DUI A; 1TXY A; 2X6O A; 2GIZ A; 2YPR A; 5KYI A; 4JBW A; 2M8E A; 2VNU D; 4FF2 A; 1MBG A; 5KVR A; 4P2C B; 3D6W A; 4XL9 A; 2HCI A; 4CJ0 B; 1J5E Q; 2MGQ A; 2NVU B; 4U67 3; 2YSR A; 3MXN B; 2DOE A; 2NVX B; 3KEY A; 3SHF A; 3H09 A; 2PJR B; 2NAZ A; 1T39 A; 5BQT A; 2KA7 A; 1P2F A; 3ABM H; 2ZXO A; 1Z6R A; 4POG A; 2HU9 A; 3DKY A; 5EGT A; 4PG3 A; 3GFJ A; 3QOE A; 2G9H D; 3RZD H; 4WJW A; 3F8N A; 1RIO H; 4K8J A; 1YHQ I; 5EQ0 A; 1WJD A; 2BBY A; 4MHG A; 4IUT A; 2HPI A; 2L12 A; 3L2C A; 1D3Y A; 1JB3 A; 1DM0 B; 1G3Y A; 4C3I H; 4F55 A; 3CHB D; 1CF7 A; 3GP7 A; 2F6C A; 1VQC A; 1J09 A; 1PFN A; 1G8X A; 2PMZ E; 4M1U B; 2QQ9 A; 1A0I A; 3C1E A; 3D5S C; 4AH6 A; 5GQO A; 1TWG B; 4A0Z A; 1ZAR A; 4UUS B; 1LFP A; 4A3C G; 1MJ9 A; 1JKR C; 2NOJ B; 4BA1 I; 3PJR A; 1VF7 A; 4IRG A; 1FYM A; 2PNH A; 2VUM J; 3CUQ B; 1DP7 P; 3I71 A; 1C8C A; 3G71 A; 5DM7 3; 3CCJ I; 1A2U A; 2VE9 A; 4ASN A; 1B72 B; 2Q0O A; 1MBF A; 1XNQ L; 4I7H A; 2PQA A; 2BY2 A; 3Q24 A; 1SE3 A; 2ELK A; 2C5C A; 3I4N J; 4KN4 C; 4BY7 G; 3ZQI A; 1D2B A; 3OWF A; 4YIR X; 5GAE J; 2V79 A; 1XXG A; 2B2V A; 1FNV A; 2PZU A; 3U9S A; 3M4Q A; 2KDO A; 2AWO A; 1VZ0 A; 1C48 A; 5A2Q Z; 3T56 B; 5GTI C; 1K73 C; 3CXC A; 1I6X A; 2CMP A; 2BCO A; 3HYI A; 4UHV A; 2Q8K A; 5A2Q L; 1HA6 A; 4KIG A; 4DV2 L; 3E6D A; 4JI5 Q; 1EF4 A; 1ZLK A; 2LBF B; 3U1D A; 1X32 A; 3K4J A; 4K0K L; 2MBF A; 3H5R A; 1WOC A; 1LDK B; 1W7P A; 5LRR A; 1FTT A; 5IGD A; 2VC1 A; 2SOB A; 5I4D A; 1YHQ A; 2L9S A; 3DTI A; 1S6L A; 2EJF C; 2ENB A; 3K7U C; 2JEA I; 1IYU A; 5CVR A; 3V7R A; 1XG1 A; 2AXL A; 4G7H F; 2RAE A; 4FET A; 2BW3 B; 3PQI A; 2MAB A; 3C1D A; 3MEF A; 3ZPL A; 1NBW A; 1JB7 A; 4GOP A; 3ZMT A; 3S3M A; 4NBQ A; 1A2T A; 4R4O A; 3WOF A; 4FXG C; 1RTN A; 4GZY C; 1LDD A; 3C1F A; 1U9R A; 3DU6 A; 1TY0 A; 2FFK B; 2HCC A; 2E19 A; 2A69 F; 1LAB A; 4HID A; 1HXD A; 4IPC A; 3FKI H; 1IHZ A; 2DCE A; 4IUR A; 5GAE C; 1T08 B; 2E7W A; 3RPU D; 3MAQ A; 4CZC A; 1EIG A; 1ONV A; 2R7S A; 3L2Q A; 2VT2 A; 3H6M A; 3RKY A; 3PQJ A; 4Z6Y A; 4BBS G; 3FXR A; 2O2L D; 2E2J H; 4DA2 A; 3K8A A; 2E7X A; 4ME3 A; 1W6J A; 4A3K J; 2M8G X; 3H94 A; 1D8J A; 1MBH A; 3E3V A; 3M8E A; 5FD6 A; 4M8A A; 1GUU A; 4EQ5 A; 3OYM A; 3F21 A; 2P7V B; 1GUS A; 2VUM B; 3KZI C; 3GFI A; 2E71 A; 4RAU C; 1HNW Q; 1EJ9 A; 3M4P A; 2H4O A; 2V4J C; 3I4P A; 2QSG X; 5F1R A; 1U2W A; 1B8A A; 1QU3 A; 3NEM A; 4KT5 C; 3KLS A; 3BZC A; 3ND8 A; 5CBA E; 2YTY A; 2CSO A; 3ERQ A; 4JOM A; 3BG9 A; 3MKY B; 3CCL I; 1DJR D; 3JAM i; 1BI2 A; 2VPL A; 1QCB D; 4LUO A; 3HHH A; 1MD2 D; 1J2X A; 1TS2 A; 4X66 L; 2EV6 A; 2LMD A; 2HOT A; 3VFZ A; 4XK4 A; 4DV3 L; 1BM9 A; 4RG1 A; 2QW7 A; 3HRZ C; 4V19 K; 5C4J J; 1QPM A; 3G6J B; 3K70 D; 2DN8 A; 4JCV E; 4DQ2 A; 2Y0M A; 2DYR H; 1IW7 D; 3QOP A; 1T95 A; 1AOY A; 1LUZ A; 2D2W A; 3BR2 A; 1Z21 A; 5C4X J; 1RI7 A; 4CJ2 C; 4M9Z A; 2EZH A; 3MFY A; 5DLQ E; 4ZK9 B; 5IWA Q; 2K1B A; 1H88 C; 2MQH A; 3FKJ A; 2XAH A; 3I59 A; 2V7F A; 2LSO A; 3DWP A; 2R5Z A; 3DLA A; 1Q8K A; 2MJ8 A; 5F04 A; 1Q1H A; 2XWB B; 2P5V A; 1SYD A; 2TRT A; 4F92 B; 2JA8 B; 1BKB A; 2RKH A; 2E76 C; 4Y13 A; 2Y9Y A; 1B6A A; 2LKS A; 1HW2 A; 2KHM A; 1EY6 A; 1PTO C; 3NUC A; 2FJ1 A; 2LFB A; 2W24 A; 1PFS A; 2L4A A; 3ERS X; 2IA2 A; 4Y4R A; 1MKH A; 4L0Y B; 5JVH 3; 2NNY A; 4NB3 A; 2FJ2 A; 2ZJP F; 1UG2 A; 3WTY C; 3STB D; 1KLU D; 1G2S A; 2A74 C; 1POG A; 2EIN H; 2IA1 A; 2L01 A; 3RKX A; 5JFR A; 2M9H A; 4M6Y A; 2K7L A; 1TKW B; 4M6X A; 1R9T J; 3CCL A; 3HOW J; 5HS8 A; 3NQT A; 3Q0U A; 4MFD A; 5FKA C; 1BW5 A; 1XB4 A; 4A3K B; 1D8L A; 4FIS A; 1HRJ A; 1OKR A; 2ZSH B; 2JSC A; 2NVT H; 5FK9 C; 1SRO A; 4I09 A; 1AZQ A; 3UDG A; 3HGB A; 2KEE A; 2R0Q C; 3CJR B; 2K86 A; 2V9V A; 3KF8 A; 4G4K A; 2W48 A; 1EZ6 A; 2R61 A; 3K7A J; 3OYA A; 3SGI A; 3QO2 A; 5CVJ A; 1KDC A; 4LIV A; 3HIF A; 1XPO A; 5EPK A; 3RQ4 A; 1PTO B; 1TW2 A; 4BU2 A; 5EF6 A; 5DAU A; 2OQG A; 4LMY A; 2CCZ A; 2L5Q A; 2ZXI A; 5C44 J; 3ZN1 A; 5H20 A; 4GDF A; 2JSO A; 3FXQ A; 3BZD B; 4YG2 F; 1DDN A; 1PFM A; 4LF9 L; 1FZP B; 2M00 A; 1WJJ A; 4JOI C; 1T33 A; 3HBL A; 1YHA A; 4DNT B; 5E8G A; 2DDA A; 2A8V A; 4LIT A; 1E2Z A; 1HW1 A; 4FDB A; 2IX0 A; 4AUX A; 4DU9 A; 1W4M A; 3I54 A; 2B9S A; 3CMM A; 4RGX A; 2O4X A; 1D5Z C; 5C4J B; 1DXM A; 4EM1 A; 1RES A; 3PIO F; 3GTL H; 3JSP A; 2UUC L; 2DO7 A; 4JI3 L; 4OIK A; 1HCR A; 4QJF A; 2VE8 A; 4PQX A; 3OMC A; 1ENF A; 2UXX A; 1TWF B; 3RDI A; 1T6S A; 5C4X B; 2ELJ A; 2AUK A; 2WII B; 3A0B C; 3E5X A; 2HOE A; 2JUC A; 1XNQ Q; 2PMU A; 2XHK A; 1FNN A; 1XC5 A; 4PFO A; 5JLW A; 4CH7 A; 2Z9H A; 3TDZ C; 3M07 A; 2G9D A; 5HDN A; 2PW5 A; 4A3D G; 1AN8 A; 2JA6 H; 2UUB L; 2CVR A; 1UJ8 A; 3IL8 A; 1U4R A; 5E4X A; 2V1U A; 4DXG A; 2VAS A; 5IT9 c; 1YVC A; 3TS0 A; 5ELD A; 1KNE A; 2DA3 A; 3I73 A; 1YS6 A; 3L09 A; 1DON A; 3Q0A A; 1SYF A; 4RCN A; 5D8L B; 4E6K G; 5LMN L; 1Z7U A; 2Y2Z A; 2A5H A; 1OXV A; 4DV2 Q; 2XKP A; 1N77 A; 3HPH A; 4LGW A; 3OW7 A; 3HHG A; 1LEB A; 1MZB A; 4K0K Q; 3EA6 A; 1PYS B; 5IP6 A; 1AWC A; 1UHS A; 3AYH B; 4C2M B; 2EVB A; 4LW1 A; 4UY8 C; 4OOI A; 5DEH A; 1YRN A; 2KP6 A; 4CCL A; 2WWY A; 3ZXS A; 1JOO A; 2EY5 A; 1AEX A; 1F2L A; 2E75 C; 4C2U A; 1D8K A; 2WAQ B; 3QOJ A; 1P8L A; 3LSI A; 1C0A A; 2F0T A; 2ZO4 A; 1LVK A; 2KJC A; 3SHL A; 5CVV A; 1K28 A; 1PH9 A; 1P4W A; 3VYS B; 3LSG A; 2NZH A; 2MH9 A; 4IPH A; 5C44 B; 4DV5 L; 2GXG A; 3K4N A; 1NH3 A; 1WTR A; 3MKZ A; 3BG7 A; 4DR6 L; 4A3E G; 2JXM B; 2VZA A; 5DCF A; 3HGG A; 2E2H B; 3RZO H; 3A03 A; 3KF6 B; 1FYC A; 1TII D; 1EIY B; 3S2H B; 2PQE A; 2LG4 A; 2VQH A; 1ON1 A; 1QQI A; 4DNR B; 2H1E A; 4LLG F; 5EAY A; 3HOS A; 1SRU A; 1RL2 A; 2HWV A; 2XF7 A; 1TWA H; 1TS4 A; 3EUH A; 2FD5 A; 2WKD A; 2QKL B; 3GDE A; 1MI2 A; 4K5V A; 1F2Z A; 3I0T A; 2DFH A; 2VBZ A; 1WTV A; 2ZJR A; 1BBW A; 4IUQ A; 2FRH A; 4U0V A; 1RUO A; 1D1B A; 1EYG A; 2TDX A; 3WU2 C; 4TSS A; 4TV7 A; 3W6K B; 3PMF A; 3BJ6 A; 2GA1 A; 2DDB A; 3V96 A; 3MXG A; 5CVU A; 4BY1 J; 2K01 A; 2K9M A; 1WGX A; 1ZLJ A; 4X66 Q; 5B5E C; 1B59 A; 3OL4 A; 1L0Y B; 5LMU L; 4G6D A; 2WIN B; 2IHE A; 4A3L H; 5AON A; 3MAJ A; 4DV3 Q; 2FXQ A; 2F0M A; 2X51 A; 2K9L A; 4HX7 A; 1EY8 A; 1FOX A; 1RKT A; 2Y0S G; 1XN7 A; 2JT1 A; 2WAC A; 3O6B B; 1I1S A; 1R1V A; 3D5R C; 3OSO A; 5KGU A; 3OYF A; 1XX5 A; 2ESH A; 4A5M A; 1J8I A; 2F5E A; 2OCE A; 4G7O F; 4O2D A; 4P9F A; 1K78 B; 4IUU A; 1FGB D; 2PG4 A; 4I76 A; 2UXN A; 3W2A A; 1D0X A; 4QL5 A; 5IP7 H; 3KEQ A; 4HA8 A; 1T8I A; 2WAE A; 1VQH A; 4Y7N B; 1UAP A; 1F5T A; 2KL4 A; 1PFB A; 3TP6 A; 5IT9 E; 1GKH A; 5IP9 H; 3ULQ B; 4A3G G; 2KIF A; 2Y0S N; 2M0C A; 3SZT A; 4L5T A; 2L7Z A; 3FF5 A; 3Q3S A; 2VUM H; 1GD7 A; 5EJW A; 1X2M A; 2KOL A; 3MFG A; 3I4M J; 2QA4 I; 2D9U A; 1EZ8 A; 5D5W B; 4ZQ3 A; 3GF2 A; 5IY5 H; 1K0R A; 1VMC A; 2QDL A; 1W2B A; 2KHI A; 4CDG A; 3BCA A; 2E1C A; 4D7M A; 4MHE A; 4FXK C; 2NVQ J; 3H36 A; 4WJ4 A; 3QRF F; 1CKN B; 3I4N H; 5K7H A; 1BNZ A; 2EXZ A; 2K3P A; 2PEX A; 4FNF A; 3QU6 A; 4BHP A; 5ILU A; 2B2Y A; 1X3Q A; 1XPR A; 1N75 A; 4X1E A; 4JI1 Q; 5C3W A; 1T0F C; 3JTG A; 4CGZ A; 4RHR A; 4HDV A; 4D1Q A; 1DZ1 A; 2RSN A; 2VB6 A; 2K5Q A; 1I3Q J; 2DY7 A; 5HOO A; 1NTC A; 4POF A; 3DU5 A; 3KE2 A; 4LF8 L; 2A61 A; 4BQA A; 1FX7 A; 2QYO A; 2PN6 A; 1XSV A; 1T5E A; 3G1M A; 4KJO A; 3D4D A; 4CYC A; 3FDT A; 3AEV A; 3C2P A; 2M3A A; 2DI3 A; 4YFX F; 3HOX H; 2STW A; 3MCZ A; 2DA1 A; 3DLL A; 3F8T A; 3WE2 A; 4ZCK A; 1G2T A; 1PZJ D; 2RVL A; 3I55 A; 4IN3 A; 1R7J A; 3UR3 C; 3A74 A; 5IGC A; 2V1N A; 1HZB A; 4LF9 Q; 1B1Z A; 2Z8L A; 2ZKZ A; 4B08 A; 3WGH A; 3UDI A; 3O3K A; 4HBL A; 5EEH A; 2AQF A; 4MIM A; 1BW6 A; 3OYD A; 4FYD A; 3K4K A; 2KT0 A; 2A68 F; 2F48 A; 2EYF A; 3OYE A; 3R61 A; 5C4H A; 4HZF A; 4JI3 Q; 1UWV A; 2GBY A; 2LLK A; 1EY5 A; 1WZ2 A; 1KL9 A; 4TVA B; 2Y0S A; 5IYC J; 4PGG A; 4QTR A; 3VYT A; 5KIX A; 3BJA A; 3MVV A; 4ZLT F; 1RD9 D; 4IFD G; 2FUG 2; 3K1N A; 2OC6 A; 4LFC L; 3T0Y A; 1PP8 F; 2AHQ A; 1UST A; 3OOC A; 3HRS A; 2CV1 A; 1LTB D; 4A3K H; 4UB6 C; 2FA5 A; 3H5A A; 1FY7 A; 4C3H B; 4ZYG A; 1MMA A; 2WAQ E; 3NPP A; 5J8F A; 5D3I B; 2UUB Q; 1AE2 A; 4C30 A; 5F0F A; 4RCO A; 2JRT A; 2HFH A; 4U87 A; 1VFC A; 4IHV A; 1S72 I; 2ECC A; 4Q48 A; 1RRJ A; 1BCP D; 4PFP A; 3I4M B; 2HKO A; 5IYD G; 1EV7 A; 5C0X I; 1PTO H; 4ZYH A; 2V6E A; 1K0S A; 2HDM A; 3K80 C; 4HQE A; 3DV8 A; 2NVQ B; 2IS4 A; 3TZU A; 5D4R A; 2QGQ A; 5LMN Q; 5CV5 A; 4BUP A; 4ZUJ A; 1HR0 Q; 4HJ8 A; 1HHV A; 3TO7 A; 3Q0W A; 1PJR A; 2ZTC A; 2QZ8 A; 3IRB A; 2EIM H; 1Q1E A; 4A3B J; 3QYE A; 5AIP A; 2CV0 A; 2K02 A; 5EKL A; 3BG6 A; 4EJV A; 1X58 A; 5LEJ A; 1PYW D; 1I3Q B; 4FTH A; 5C4J H; 2EZI A; 4GCV A; 3J80 E; 4J19 A; 1Y8Q B; 3FXO A; 4BDZ A; 5BPD A; 3F8M A; 2JJ7 A; 5H6R A; 4HNU A; 3I2Z A; 2E1O A; 4A3M B; 4XQJ A; 3HRU A; 1VQO I; 5EDN A; 2DA5 A; 1R6R A; 2YS9 A; 3HTS B; 5C4X H; 3TOB A; 1JWM D; 3CD6 A; 2FQ3 A; 2IT0 A; 3SK4 A; 1QUQ B; 3GV3 A; 2M5W A; 1B4O A; 1PUF B; 5KAF C; 4K6L A; 4KLK A; 1GUN A; 4PSX A; 1DCZ A; 5FFX A; 2CU7 A; 2KRF A; 1LE8 A; 2JA7 G; 4DR6 Q; 4DAY A; 2OQR A; 4R8N A; 5IOC A; 2HUG A; 5HOD A; 3DEW A; 1IF1 A; 4KN4 X; 1YW8 A; 3L00 A; 2Z2S B; 2VN2 A; 4UVC A; 2ID0 A; 4D5C A; 4L0Z B; 3I70 A; 3GTO B; 3IUO A; 3WTZ A; 4HSR A; 4QMG A; 1P7J A; 4P1W A; 1YJW I; 4LG0 A; 4R4T A; 4RA8 A; 4BBR J; 3R3O A; 4MTE A; 4HCS A; 4E4H A; 1X4O A; 1HFN A; 3IKT A; 2AS5 F; 3KP7 A; 4NXM L; 3REO A; 4CDD C; 1B8I A; 5EGO A; 3HOU G; 2VOO A; 3KP2 A; 1R9T H; 4ZKC B; 3HOW H; 3CQZ J; 1DPR A; 5E1X A; 2OQK A; 5J4Y A; 3FM3 A; 3RJP A; 2JA8 G; 4HJA A; 2JEB I; 3W2D A; 4IRH A; 3HOY B; 2XWJ B; 4XKC A; 1WQ2 A; 2BW3 A; 3VDY A; 1KU3 A; 4AVP A; 5LMU Q; 4CCM A; 3K7A H; 2NVY B; 2FU4 A; 4KTA A; 4MGR A; 1FP1 D; 3ULJ A; 1IZ1 A; 1EEI D; 3WTU C; 1XMK A; 1ESR A; 4ZH4 F; 1EIH A; 1Y69 U; 4YRV A; 2J7Z A; 2Y9Z A; 1JJC B; 1WI5 A; 3OYY A; 3C2B A; 4RFB A; 3CRL C; 5J47 A; 1ZYR D; 2QLZ A; 3G3Z A; 3EDP A; 3C46 A; 1MKM A; 1IGN A; 1OXS C; 5BPI A; 4I0A A; 2KMU A; 1FNW A; 5C44 H; 1JT6 A; 1IRX A; 4U0W A; 3KLO A; 2LF8 A; 1HH2 P; 1PRT C; 2ICW G; 2D5V A; 4DXF A; 9ANT A; 3ZQG A; 1O4X A; 4EJT A; 5BOX A; 1YNJ C; 3TGN A; 2F0U A; 4PJ0 C; 2CW0 D; 3K69 A; 4N4O B; 2ZT9 C; 4OO1 G; 4KMU X; 4YCU A; 4KV6 A; 4GNX A; 1VQE A; 5LFA A; 1AH9 A; 2OT2 A; 1VQ8 I; 3J81 c; 2EY1 A; 1SMX A; 5ILS A; 3K1P A; 2HXI A; 5HDK A; 1BI1 A; 2LF7 A; 2DTR A; 3KLW A; 1PMR A; 3Q9V A; 1CKO A; 3S1M J; 1AW7 A; 1ZGA A; 2A6H C; 2AQ1 B; 3PO3 J; 1I5Z A; 4NPS A; 1TS5 A; 3KZZ A; 2EWN A; 4CCJ A; 2RQX A; 4XBF A; 2WKC A; 1V1P A; 2VL8 A; 2WB1 G; 2ADU A; 4L8J A; 4XOT A; 3CCU I; 1OXU A; 2HT1 A; 4EEF G; 2K40 A; 3ZYY X; 5J3L A; 4B4C A; 4KYW A; 3HOZ J; 2Z6K C; 3TQN A; 1PH1 A; 2Z5U A; 1XTD A; 4HJ9 A; 1IBL L; 1KRT A; 5JHF A; 2L9R A; 1W6K A; 4X64 L; 3F0C A; 3M8J A; 4BBR B; 1WCM J; 1CT1 D; 1FMV A; 3IKJ A; 2FBH A; 3J80 K; 4CHU A; 2JPB A; 4A93 J; 2WB1 N; 1PH6 A; 2VJ4 A; 4MIU A; 1MBJ A; 1ASZ A; 1WJF A; 4LF8 Q; 1I3A A; 3JRE A; 2QQA A; 2EYM A; 1Z77 A; 1L2F A; 3I90 A; 3OW2 A; 3TRZ A; 1Z67 A; 1QA6 A; 3HS0 C; 4GS3 A; 5EYR A; 2CFX A; 2E74 C; 2DFE A; 1FEX A; 2I0Q A; 1RNL A; 2R92 B; 1RCV D; 4B3S L; 2IA0 A; 3FRP B; 2VOD A; 4OXP A; 1OQY A; 3RCO A; 4HTH A; 1BOA A; 1JE5 A; 1YJ9 I; 5JVG 3; 2A73 B; 2F0V A; 2GXA A; 2DN0 A; 1MIU A; 3WTT C; 2NNN A; 5IYB J; 2CQO A; 1NG6 A; 4YFK F; 2QKM B; 1YS7 A; 2MP1 A; 1CHQ D; 2PX9 A; 2PW7 A; 3LSJ A; 5FO5 A; 3ERE D; 4AYB B; 2EFN A; 2BYV E; 4KHP L; 2XRS D; 2JA5 G; 4DK6 C; 3EIV A; 3BWG A; 1QBJ A; 5FLM G; 5DD8 A; 3CCU A; 1E3H A; 1VQ6 A; 2DNE A; 2DJG C; 4LFC Q; 4ZTJ A; 3HM5 A; 4M73 A; 4PJL A; 3UDX A; 1YI2 I; 5HNH A; 3FKI G; 1Z91 A; 1PH3 A; 3V8L A; 1JGS A; 3S1M B; 1MJB A; 2E2I J; 1ACI A; 1WVR A; 4MN3 A; 3UI2 A; 3WGG A; 4LIU A; 1V5G A; 2C6Y A; 2JZY A; 3A8I E; 1MJE A; 2JA9 A; 4HNT A; 2BXZ A; 4A3F J; 5D8K B; 1K83 J; 1Y1W J; 2O5I C; 4L9T A; 4NDX A; 4EVI A; 3G4S A; 2H94 A; 2VXD A; 5DM6 F; 3HOZ B; 3TP8 A; 1YIT A; 3QP6 A; 1FNU A; 2XGC A; 4BY1 H; 3S1Q J; 1HC8 A; 1E0E A; 3J81 E; 1D1A A; 2WB1 A; 5C0X J; 1STY A; 1HQR D; 2FX0 A; 2ZCW A; 1ETX A; 1A31 A; 4JYA L; 5J1Y A; 4FE7 A; 1IG7 A; 4I02 A; 1OCP A; 1VQN A; 2QF7 A; 4GN4 B; 3Q9S A; 3MXP A; 2NB0 A; 1YJ9 A; 2H5X A; 4HLY A; 1JKP C; 5IOZ A; 4A93 B; 4I0B A; 2X6W A; 1YBY A; 1SAN A; 1NE3 A; 5M3F G; 4BL0 B; 1BJZ A; 4HOB A; 1N78 A; 2EYL A; 2NRA C; 3BR1 A; 4E7J A; 2A69 D; 2IPQ X; 1Y8P B; 4ODG A; 2N3S A; 3RYR A; 3T13 A; 4YWL A; 2XKO A; 5FO7 B; 3TKY A; 3H0G B; 1EQQ A; 4HMJ A; 3M7N A; 1CF7 B; 1SFK A; 1V4R A; 3S1N H; 3AG2 H; 4KUM A; 1E2W A; 1BBU A; 3CCM I; 3TBI A; 2KRC A; 3K5L A; 3OR2 C; 4IF4 A; 1TQP A; 1YI2 A; 2A5W A; 4IQ4 A; 4OON A; 4LB6 B; 1S5E D; 2ISY A; 2JPC A; 1X3W B; 1ODD A; 1RDP D; 3TS2 A; 3I4M H; 2OTL A; 2M7B A; 1I4Q A; 2B2T A; 2F0S A; 1TY2 A; 1I4X A; 5CGK A; 3SK5 A; 2F0W A; 1U8R A; 1MIJ A; 1YW9 A; 1SNC A; 1IYJ B; 4A3C J; 4QSH A; 3PQK A; 2NVQ H; 3AQQ A; 3ZP5 A; 3OV8 A; 2VXZ A; 2D45 A; 3TN2 A; 2PQA B; 4NXM Q; 2KIU A; 3BJO A; 2A19 A; 3GTQ B; 1OCR H; 2X4H A; 1A62 A; 5FKN A; 3TOA A; 1Q8I A; 3ONA B; 1CQF A; 2XPU A; 2K4K A; 2QSF X; 4GOP C; 2Q1Z B; 2EFO A; 3T1Y L; 1BCP B; 4MEY F; 1O7Z A; 4KKT A; 3FIW A; 4GKK L; 1KA8 A; 1KYW A; 2WP8 J; 4F3Q A; 2E2I B; 3TW7 A; 4YY3 L; 3DBH B; 2LY2 A; 3FMS A; 2P6T A; 1K83 B; 1MDM A; 1Y1W B; 1HKT A; 2JJQ A; 2C35 B; 2VB2 X; 1D0Y A; 3OYJ A; 2RF4 A; 3LA2 A; 3GTJ J; 1W7P B; 1YE9 A; 1MMD A; 1YJN I; 3NBI A; 4M9S A; 3MQ0 A; 5L3B A; 2R5Y A; 2HDC A; 3W5O A; 3ABU A; 3PUF A; 4CUG A; 2HOS A; 4A6D A; 4NMZ A; 2M87 A; 5LL6 S; 4Q0F A; 1UAA A; 2LTP A; 3DXJ C; 3LSH A; 3GTM J; 4EQQ A; 1ML0 D; 4ZYA A; 4KD3 A; 2P5K A; 4II2 A; 1ETQ A; 2H27 A; 3GX4 X; 3NSW A; 2CH0 A; 1U6G A; 1UE7 A; 5IT3 A; 1GO3 E; 5D6E A; 1EWH A; 2K7V A; 3HFZ B; 5K5Q A; 1W36 D; 5FJ8 H; 1YIJ A; 4A5N A; 2R7R A; 2BE5 C; 5IYC H; 3TP7 A; 1F4K A; 4S04 A; 1FOW A; 4JX6 A; 2EIJ H; 2YR2 A; 3PV0 A; 2VON A; 2CFM A; 3K4M A; 3H1C A; 2XNA C; 3CCS I; 2JWT A; 2NTT A; 1LTG D; 4BH9 A; 1Y0U A; 1SE4 A; 3LST A; 2NN6 I; 2W84 A; 5DD4 A; 1U0J A; 4RM2 A; 4MO7 A; 1EH8 A; 2M7A A; 2AQE A; 4XT3 B; 2OF1 A; 1HDP A; 2E1M B; 4R1J A; 1XMO L; 5CQE A; 2DK5 A; 3LL7 A; 2N49 A; 2I07 B; 2JUG A; 3S14 J; 3DEO A; 3OMG A; 4X64 Q; 3CO6 C; 3RPQ A; 4DBR A; 4HX4 A; 1HA8 A; 3CRK C; 5ERI A; 1FTZ A; 5LHI A; 3PO2 J; 2JA6 G; 2F5D A; 3BVZ A; 2H8R A; 2DFI A; 4P55 A; 2L0K A; 3ZQL A; 4WOR A; 4WFN 3; 3CC4 A; 3OOP A; 4A3B H; 4B3S Q; 4EX5 A; 2HTJ A; 3NRV A; 2HZT A; 4BY7 B; 2B63 G; 1EY4 A; 2F5C A; 5M5Y J; 1UE5 A; 3ML6 A; 1J7K A; 2MO0 A; 2RN7 A; 1UMQ A; 5JJL A; 4MQ9 F; 2K3F A; 1F9P A; 4I6Z A; 4ZDO A; 1Y39 A; 2UXD L; 4RH6 A; 4AIH A; 2OBP A; 3W6J B; 2VW9 A; 2QIL A; 2VQE L; 2Z3Y A; 3WGI A; 4DV1 L; 5TIS C; 3HKS A; 3G6E I; 1HW5 A; 3ABK H; 1YNN C; 4ID6 A; 2NS0 A; 1CZG A; 2B0L A; 2KKO A; 2MFZ A; 3I59 B; 4RB3 C; 1ZR4 A; 3IHU A; 3GTK J; 4PMW A; 2WAD A; 3JRA A; 4KHP Q; 4C2T A; 2WBM A; 1D5M C; 4WXD A; 2R5Z B; 3KIO A; 1FOY A; 4YIJ A; 2ACJ A; 4NW2 A; 3CCS A; 1TTY A; 4ESF A; 4Y52 J; 1P92 A; 4A6E A; 4CKB A; 1KHI A; 4P74 A; 1EU3 A; 1OJL A; 3GTM B; 3K1M A; 4EJU A; 2VI6 A; 1PHJ A; 2YTX A; 3P7N A; 2I46 A; 3GTG B; 4BBR H; 2CH4 W; 5M3M J; 5CYV A; 1SYE A; 3IP4 B; 3AFH A; 2V4D A; 4D5F A; 2IGN A; 4EQO A; 1DU6 A; 3OIO A; 4JI7 L; 3CJT B; 2ZJ2 A; 3R0J A; 2IGO A; 3I53 A; 3V7S A; 2L55 A; 3WHB A; 3CQZ H; 2Q2H A; 1EL0 A; 4WX9 A; 2Y48 A; 3KK7 A; 1N33 L; 3OPO A; 3K2A A; 1CKM A; 4B3M L; 4IUP A; 2V26 A; 2QKI C; 3ZMS A; 3HP3 A; 4KIC A; 1IBM L; 4BBS B; 1XPU A; 2XVC A; 2PQ8 A; 4JBJ A; 5EBC A; 1RTO A; 3KF8 B; 3S14 B; 4JYA Q; 5K1Y A; 4D9J A; 3EQL C; 1XDS A; 1HZ9 A; 2JK3 A; 3M1C A; 4A3I J; 2JYO A; 3PUY A; 2OJE D; 2CS3 A; 3I8Z A; 2BDN A; 3OR1 C; 1PZK D; 5ELF A; 1IJW C; 1CK1 A; 4A3L G; 3D6C A; 3P75 A; 4K5X A; 3G6E A; 2X6G A; 4FF1 A; 2UXB L; 2B2U C; 2R6G A; 2DBB A; 3LWU A; 2CRG A; 4IO9 F; 5C5M A; 3T8R A; 3U50 C; 2VBW A; 2HR3 A; 1G3W A; 2Q8I B; 2CGP A; 1SNM A; 1GHJ A; 4JI4 L; 3WTW C; 1S3O A; 5JJZ A; 3S15 J; 2CUE A; 1BBX C; 1CA6 A; 4DIQ A; 5NUC A; 1MSE C; 5IP7 G; 3KBX A; 2OUM A; 3PO3 H; 2UXC L; 3WWV A; 3M4O B; 1PTO F; 3S17 J; 4KY5 A; 4U7B A; 1P9Q C; 4RGS A; 3ZMD A; 4ANJ A; 4PSW A; 2JVG A; 4DK3 C; 5IP9 G; 1IRF A; 2YTV A; 4XLP F; 1PXU A; 1GM5 A; 3GTK B; 2EYH A; 5FGM A; 2V9P A; 2L0C A; 1TQI A; 2SNM A; 2AWN A; 1TWH J; 3UE0 A; 3EJI A; 2MD5 A; 3DM1 A; 2M0M A; 1Y0N A; 2F0G A; 3M85 A; 1Q7Y C; 5DCM A; 1VQ5 I; 3Q6S A; 5EKI A; 3T1Y Q; 1WCM H; 3I4N G; 4GXO A; 1Z6H A; 4GKK Q; 3T1H L; 2VKD A; 1OO9 B; 3JW4 A; 2IX3 A; 1G29 1; 2LY1 A; 2KHS A; 2W00 A; 2IP2 A; 3GME A; 4AID A; 3IL2 A; 2COM A; 3SZP A; 3SYT A; 1GJX A; 2Y30 A; 2BH2 A; 2P5Z X; 2HQN A; 3R60 A; 4XOR A; 4K14 A; 4ENN A; 5M5X J; 3IKV A; 2VOP A; 2JTV A; 1FVI A; 1LTA D; 2EE1 A; 2IRF G; 4CJ1 B; 2ICE C; 3R8B A; 3PUB A; 4X6A J; 1YRN B; 3BPV A; 2MC3 A; 2XUB A; 4HV6 A; 3F2U A; 1I4R A; 2VKH A; 3EUH C; 2ZSI B; 1SYC A; 1VQP A; 5D14 A; 1S8N A; 3RYP A; 4EJO A; 1G4D A; 4J1M A; 1TNS A; 5EBD A; 5F08 A; 3VA5 A; 2X0L A; 4IVN A; 3HOX G; 1FOK A; 2K50 A; 5IYB H; 1RET A; 4R1H A; 2G0E A; 1VQ7 I; 3EN2 A; 1EYC A; 1LJ9 A; 4AIJ A; 1U3E M; 4DAP A; 5J8C A; 2EYP A; 4FF3 A; 4ZZD A; 3DEU A; 4ENK A; 1R4M B; 4OIO F; 1LPQ A; 5CV4 A; 2BE5 D; 4RGU A; 3ERO A; 3T16 A; 5ILV A; 1EYA A; 4I7Z C; 2BOL A; 3TEH B; 3DP7 A; 3AG1 H; 2ECB A; 4FF4 A; 3BVM A; 3B73 A; 5L3D A; 4K2L A; 3OSG A; 1AA7 A; 4JI8 L; 2X48 A; 2XPV A; 1EFI D; 4UAI A; 2A3S A; 1XS9 A; 4IOC F; 4KKS A; 5B3S H; 2JP1 A; 2HIV A; 3FMQ A; 4IJL A; 4HX8 A; 1L1O C; 4GLV B; 1WFX A; 4RDU A; 1Z6T A; 3S15 B; 1B3Q A; 5JEA J; 3RLO A; 1XMO Q; 4L5E A; 1VQ5 A; 2E2I H; 5D5V B; 1BBY A; 4HJ5 A; 1ZG5 A; 4PV1 C; 2AUJ D; 4AYB G; 3ZMZ A; 2VLA A; 1GT0 C; 1MJA A; 4JI5 L; 2TMP A; 2YU9 H; 4J8Q A; 2ZXJ A; 2KFD A; 2WTE A; 3GFL A; 4A3F H; 1K83 H; 1Y1W H; 3HEJ A; 4RAG A; 1BC7 C; 1KLG D; 1S5B D; 4GKJ L; 1LM0 A; 1TWH B; 3S1Q H; 2BY1 A; 5KNL A; 2SNS A; 4HNV A; 5C0X H; 2DJF C; 2R7Q A; 4C3J J; 1X1D A; 3L2V A; 1KD1 C; 3L2P A; 4GO1 A; 5GRO A; 1KXL A; 3KP4 A; 4DZJ A; 4BS9 A; 1BF4 A; 5LGT A; 4B09 A; 1QNU A; 1F9S A; 3HSF A; 2EDG A; 4LNQ A; 4A3D B; 1SEU A; 1PH5 A; 2UZK A; 2A68 D; 3LMM A; 4AYB N; 2CH4 A; 3MTS A; 5IT9 Z; 2A6E C; 5F0C A; 3BOQ A; 2ZJQ F; 4OWW B; 3TO9 A; 2IAI A; 3K58 A; 2UXD Q; 1S4Z A; 4O0A A; 4RGM A; 5M5X B; 4DV1 Q; 1LT3 D; 2DPD A; 5IT9 L; 3V7C A; 2RDH A; 1AHD P; 3CMY A; 2B2Y C; 4XLE A; 2ZM6 L; 3WOE A; 3K5O A; 1WJA A; 2NOG A; 1STG A; 4XYO A; 1TR5 A; 2JQO A; 2F4O B; 3ASN H; 1BN5 A; 3K81 C; 1G8Z D; 5L3G A; 4QCL A; 2F0H A; 5FKO A; 2F4V L; 5D65 A; 1Q1B A; 2HQL A; 3E6C C; 2OU2 A; 4MTN A; 1RKN A; 1VQL I; 1IN4 A; 2NVX J; 1SN8 A; 1NR2 A; 1A3U A; 2FBK A; 4ZJZ A; 1U4P A; 3HZX A; 5E3O A; 3IWF A; 1IO2 A; 4A3C H; 5FFZ A; 2ZFW A; 3IH4 A; 3JRG A; 2CYY A; 3H3V C; 1KRS A; 4JI7 Q; 4QB4 A; 2XAU A; 1SVL A; 4HIM A; 2RBM A; 3E7L A; 1T2K A; 2GM4 A; 1WRJ A; 1N33 Q; 2EJM A; 2RCF A; 3BTC A; 1HZC A; 3R93 A; 1B44 D; 4A3E B; 1ONL A; 4DR1 L; 1HOM A; 2OTJ A; 2RRE A; 1W5C C; 1IBM Q; 1SEB D; 2XGD A; 2EY6 A; 4LFA L; 1AFP A; 1TWG J; 3HOW G; 2RV8 A; 4ENJ A; 4B47 A; 1VQF A; 1Z3X A; 2XAF A; 2ATW A; 3K6G A; 1YZ6 A; 3PIO 3; 4ESB A; 3EPZ A; 2GXB A; 1E24 A; 5GAD J; 3W3A A; 2XRO A; 4WCG A; 5EZH A; 1NK2 P; 4CYC B; 5IWA L; 3GTJ H; 4KI0 A; 1RR7 A; 2XB0 X; 3VSK A; 4AYB A; 3F8F A; 2DY8 A; 1QZG A; 2DOD A; 3QIA A; 3COA C; 3SDG A; 2UXB Q; 2LKP A; 1Y1V G; 3SQN A; 2VKE A; 3GD7 A; 4DPG A; 3WDN A; 3GTM H; 3TME A; 4DUZ L; 2A6T A; 1M1E B; 1V5F A; 3T51 B; 2DA7 A; 2JN6 A; 4X62 L; 4WJ3 M; 1RHP A; 1B4A A; 4JI4 Q; 4ZDP A; 2M2L A; 1I50 B; 1UG0 A; 2R3S A; 5EJC A; 2B39 A; 1XJV A; 1VQL A; 1SNO A; 2UU9 L; 2EPF A; 2ME6 A; 2NS7 A; 2UXC Q; 2Y0S B; 1AZP A; 3VYT B; 4A4I A; 5CR3 A; 3OS0 A; 4HAE A; 1ETK A; 1EY0 A; 5LEK A; 5GPC A; 3M1E A; 2ESN A; 2GOX B; 3A0J A; 3HKZ G; 1PZI D; 3KFW X; 1SFO B; 2XTI A; 3KX2 A; 3G48 A; 2VBX A; 2IJL A; 3K0L A; 2NWG A; 3H9Q A; 3K4L A; 4RAZ A; 3CCR I; 2LMC B; 4PZ8 A; 1QPI A; 3S14 H; 5GAD C; 3TDQ A; 1I6H B; 3N97 A; 4OIP F; 3FXM A; 3CUQ C; 1HLV A; 1ROD A; 3A1Y A; 2BDO A; 3F22 A; 3ZRG A; 4A3G B; 3T1H Q; 3PO2 H; 3R2T A; 3D1N I; 3M3Y J; 3J80 Z; 3OWT A; 3ZQH A; 1JI8 A; 3FX3 A; 1K61 A; 1U5K A; 1SMT A; 3R2I A; 3T1B A; 2BZT A; 2XRN A; 3HKZ N; 4IUV A; 2EJG C; 4UY8 I; 4PZ7 A; 3N6R A; 4KIB A; 3TP3 A; 1IL8 A; 3EUA A; 1E3P A; 3J80 L; 4JOI A; 2F0N A; 1KTK A; 4XOP A; 3K0Y A; 4WXC A; 4XR6 A; 3GFM A; 1ASY A; 5M5Y H; 1K3B C; 2F0E A; 5D3D A; 2E5N A; 3ZN0 A; 3UNG C; 1ESF A; 3JAM c; 5J6X A; 1JOR A; 5K7Z A; 4IHS A; 5J39 A; 3F3X A; 2PYK A; 5H6Q A; 1MSF C; 3A7A B; 3WE3 A; 1OXX K; 2R7T A; 2R7Z G; 4TQU S; 4L9V A; 2J0T D; 2O7O A; 4DGW B; 5FOB B; 1VI0 A; 3ZOB A; 3L2R A; 1MGT A; 1UEB A; 3MXU A; 3GTK H; 1M1K C; 2WY7 Q; 3QPT A; 2IW3 A; 2CQN A; 1H0M A; 1LE8 B; 1QVF A; 4IOC A; 3CCQ I; 4DAY B; 2MLG A; 5M5W J; 1KZH A; 4CCN A; 2R4G A; 2HOA A; 1X65 A; 3DQV C; 3CC7 I; 4IXA A; 4M3F A; 3KP6 A; 4JI8 Q; 3VA7 A; 4YWK A; 2VC0 A; 1JHH A; 2JA7 J; 1ZEQ X; 2F3I A; 5M3M H; 4A3J H; 4LF6 L; 1WTX A; 5CLS A; 4LG0 B; 1IDY A; 1SFU A; 4KKU A; 1JWU D; 2KQ3 A; 4HST A; 2EV5 A; 3SR1 A; 4HW0 A; 1TWC B; 2CQX A; 3JRF A; 1B8I B; 5EGO B; 3J7Y W; 4OU0 A; 5TRD A; 4B8X A; 2MHV A; 3NWT A; 4LMQ D; 3BCB A; 2RQP A; 3S2D B; 4JBA A; 2C9O A; 4GKJ Q; 5EZG A; 3KYC B; 1OR7 A; 3ABT A; 5HLG A; 1F1Z A; 2FEZ A; 3CO7 C; 1HNX L; 1F43 A; 1HKS A; 3SDZ A; 1WI9 A; 4G6T B; 4OIJ A; 1J6Q A; 3OYC A; 4GGG A; 3KYD B; 1A3T A; 3HKZ A; 5I9P A; 2XC7 A; 3I91 A; 2JA8 J; 2IGK A; 3K4B A; 2XB5 A; 2ZJQ A; 1HTP A; 4OX9 L; 4KFC A; 4N9T A; 4A3I H; 3LNN A; 3ECH A; 4BY1 G; 5DS9 A; 3M3Y B; 4ZUI A; 4H4K C; 1Q82 C; 1GHK A; 3EVQ A; 1IXS B; 1E17 A; 2M9V A; 3HTU A; 1SMY C; 1YIO A; 2UV1 A; 2VBY A; 3CCJ A; 3B6C A; 5C6A A; 1IN6 A; 1UEL B; 5CMD A; 4NB5 A; 2CQA A; 4X3T A; 3JSO A; 1F2Y A; 5LHY 1; 1DQU A; 2ZM6 Q; 4EVO A; 1KC8 C; 1HST A; 1XNR L; 1TQM A; 4GNX B; 1KAA A; 1ET6 A; 1ZRE A; 1QZE A; 4HEA 2; 1T98 A; 5FLX E; 5JI6 A; 1CGP A; 4RGR A; 2F4V Q; 4F7X A; 2QM3 A; 2X1E A; 2KZG A; 4BXX J; 4HAM A; 4J6H A; 1LT6 D; 2JE6 I; 1NCV A; 1NMF A; 2OPT A; 3G1O A; 1B9N A; 1IL2 A; 1BR2 A; 3S17 H; 4L5S A; 2D62 A; 4UHT A; 1XCB A; 1OYW A; 1X3F A; 1V1P B; 4LLN A; 4M6V A; 3BY6 A; 5KEE A; 3D8G A; 1TZL A; 5F2D A; 3K57 A; 2W4S A; 1TWH H; 4LF5 L; 5DEQ A; 1P4A A; 3NTI A; 3NTK A; 5M5W B; 1QNT A; 1IC8 A; 3ERM A; 3TL4 X; 4DV4 L; 2Z33 A; 5E7N A; 2RHS B; 2EZK A; 4YEJ A; 2JA7 B; 3MVP A; 3P9K A; 1D1K A; 3JAM E; 1PA6 A; 3F8B A; 1YO5 C; 4DR1 Q; 2CW7 A; 4XDX A; 5JR3 A; 1BQQ T; 2GQQ A; 1SDF A; 5LHG A; 4LLL A; 2PLY A; 3UDF A; 2A07 F; 3LGJ A; 3MKL A; 4BJT A; 2HKX A; 2YV5 A; 3CP2 A; 4KY7 A; 4LFA Q; 1TBX A; 4R8H A; 2Y0S E; 4N4N B; 1A3V A; 2DTZ A; 1QVC A; 4YHH L; 1TWF J; 4HJ7 A; 1D6E C; 2XSD C; 5K5R A; 5M5X H; 3QPL A; 2VJJ A; 4HCE A; 4LK1 C; 3R4K A; 1BXT A; 4UDU C; 3DTK A; 1IYV A; 2DAO A; 2F0F A; 2LOO A; 5TSS A; 4BNC A; 5KNB A; 4QLC U; 3CTA A; 4OEL B; 1PRT F; 5LMN W; 4X6A H; 5HS9 A; 3HOU B; 4F87 A; 1PLF A; 2EFW A; 3GTP B; 1CHP D; 3HOV G; 1C4Q A; 2K6A A; 2AO9 A; 3QYB A; 3Q5F A; 2R93 H; 1DU0 A; 2MUY A; 4OWR B; 4EJW A; 2HGC A; 2F3V A; 2MG4 A; 4OIQ F; 4ON0 A; 4DUZ Q; 4GRI A; 1G59 A; 4N9P A; 4G57 A; 4X62 Q; 3I3L A; 1FJL A; 2D2C C; 3PNY A; 2JSP A; 2DJI A; 3OOU A; 3EBE A; 3VR4 A; 3OSF A; 1X2N A; 3E6B A; 2UU9 Q; 3CU7 A; 2A6H F; 1VQ9 I; 2E60 A; 1R22 A; 3LSP A; 4DNC A; 1QHH D; 4OMY A; 4OMZ A; 3K0X A; 4KD4 A; 1WH5 A; 4V0B A; 3GQB A; 4U7D A; 5A35 A; 3BP8 A; 4W97 A; 2IX1 A; 4MLO A; 1JE4 A; 2RGV A; 4E6I A; 4JI1 L; 3A2K A; 4JV5 L; 3WU0 A; 4C1N K; 5FJ8 G; 1O7Y A; 4C2M J; 2JA5 J; 4XRS G; 5JEA H; 4RLF A; 1XMQ L; 3DBL B; 2KVM A; 4XSX F; 5K36 G; 3GWZ A; 3J81 Z; 5FLM J; 2K5H A; 1LFU P; 4FP5 D; 1Z3Y A; 4CZZ A; 3KP3 A; 1KU9 A; 2NN6 H; 3CAM A; 4R4I A; 4FX4 A; 3RZD B; 2P81 A; 1Z9C A; 4RKL A; 4XRM A; 3QOD A; 1JHF A; 1IN7 A; 1OPC A; 2ZXP A; 2MA3 A; 1C7Y A; 3J81 L; 2GAU A; 1S7O A; 4Y3O A; 5JAV A; 1V1O A; 4DK1 A; 2EZL A; 2KCT A; 4WRD A; 2LK2 A; 2DLL A; 3FEW X; 4XLN F; 4M72 A; 3JRD A; 3D5L A; 2IY5 B; 1K8A C; 1JMC A; 4C3J H; 2J5P A; 2WB1 B; 2E2H J; 1JIC A; 2F0D A; 2DW4 A; 3BJU A; 2NXN B; 2A05 A; 4TRD A; 1V5E A; 3S2H J; 1SE8 A; 4G7Z C; 2W57 A; 4HSP A; 2B2T C; 3H15 A; 4BQO A; 2BEO A; 1O7L A; 1JJ6 C; 5FLX K; 1ORK A; 5J4V A; 5GAG J; 4A3B G; 3O8H A; 4RKB A; 5KYL A; 2VS1 A; 4BYY A; 2AMC B; 2PNR C; 5E8I A; 3PUW A; 2FXA A; 1E2V A; 1Q86 C; 1BOB A; 1XMA A; 1STB A; 3BDD A; 4E7K A; 2W1O A; 1X3Z B; 5M3F J; 1I5F A; 2BA1 A; 2H6C A; 4AQY L; 4DAV A; 4JPB W; 1HZA A; 2RDG A; 5CV6 A; 2HPM A; 3H3V K; 5E3F A; 4J15 A; 2VIX A; 1WSU A; 4NC8 A; 1D7Q A; 1IKL A; 3RN2 A; 4MNU A; 3TBI B; 4EGV A; 4DYQ A; 1Y14 B; 2Z9O A; 1IAW A; 2NVX H; 3HRT A; 1G2H A; 3JAM K; 2I5H A; 1TQO A; 2L9N A; 4LF6 Q; 1DYQ A; 2MO1 A; 4EV0 A; 2KDZ A; 4GYI A; 1QGP A; 3E6Z X; 4RB0 A; 1HR0 L; 2LVS A; 1ETY A; 2HXO A; 4BKX A; 1ZG3 A; 3OYI A; 1PYV A; 2KED A; 5J3B A; 2E35 A; 3FMC A; 2OWO A; 3KBG A; 2R7O A; 3K5M A; 2JA5 B; 1D1C A; 5EG0 A; 4B44 A; 2X85 A; 2Q2T A; 2OF7 A; 4Y7N J; 4DAM A; 3LQR A; 2B7E A; 3WG7 H; 5L3C A; 1DOK A; 5GAG C; 2NZ1 D; 4JW0 A; 1HNX Q; 1JQY D; 4BA2 I; 1NAP A; 2VKV A; 1TWG H; 2ZC2 A; 3E5U A; 2WV6 D; 4KA4 A; 1JTY A; 4XMY A; 2UWM A; 2CO5 A; 4PCQ A; 4XRS A; 1KYZ A; 2OXP A; 3FKI B; 4OX9 Q; 3FK6 A; 3OTO L; 1XTA A; 4BE2 A; 2QQB A; 1FT9 A; 3RQI A; 1MDM B; 4RAY A; 1X54 A; 1SD4 A; 3FM5 A; 2K4B A; 2E2J B; 1PDQ A; 5LHH A; 3LAP A; 3WHC A; 3BTJ A; 2R5Y B; 3M9B A; 3W2W A; 4APV A; 1JT0 A; 1O78 A; 4AU7 A; 4IHW A; 4DK0 A; 1F9Q A; 4G6Q A; 4C9Y A; 4I01 A; 3CC2 I; 4A69 C; 1UZC A; 3F6V A; 4DKA C; 1XNR Q; 4OWT B; 1ETW A; 1K7A A; 3CLO A; 3U4Z A; 1IXR C; 2LRE A; 1TVX A; 3T4A C; 1JKO C; 1W0T A; 2E36 A; 3MZY A; 3NWS A; 2FSW A; 5FD5 A; 2FF4 A; 1NK3 P; 4ETS A; 2W82 A; 3SEB A; 1ILQ A; 5FKM A; 2SDF A; 3DTE A; 1S5C D; 1SVO A; 2ZME A; 5M3F B; 2P0W A; 3QPH A; 3DKX A; 1XWE A; 2DT7 B; 4LF5 Q; 2O5R A; 2JRM A; 3PO3 G; 4RBO A; 1Q3L A; 1VQM I; 4I98 B; 1Q90 A; 1B7Y B; 2EBI A; 1VQA A; 4DV4 Q; 3DBR B; 1UNS A; 4DUY L; 1KQ8 A; 1HCZ A; 4LD5 A; 1FR3 A; 1I27 A; 2DYS H; 3ND9 A; 3OS1 A; 2NYZ D; 3I4L A; 3M3Y H; 5J1U A; 1U8B A; 1TL8 A; 4UP9 A; 2QGU A; 3QWL A; 3CCV I; 4XQN A; 1STZ A; 2IGM A; 5E18 F; 1R00 A; 3DKW A; 1B70 B; 4YHH Q; 3DEE A; 3SXY A; 4L5R C; 1WCM G; 2CUZ A; 3R6S A; 4A12 A; 3MKW B; 2H1L A; 2WV0 A; 3A5U A; 1I4G A; 4HYE A; 1Z7T A; 1HSJ A; 4OWX B; 2J4X A; 2K5C A; 1BL0 A; 1E7Z A; 4IHY A; 2DA6 A; 3CC2 A; 3E5S A; 2EIK H; 1A63 A; 4K2K A; 4C3H J; 1TW3 A; 4JBK A; 4N9I A; 5KHD A; 3DXJ F; 3VR3 A; 3JRC A; 5CV7 A; 3KEO A; 3GXO A; 2ZJP A; 3ULL A; 1MMG A; 3U9O A; 3HPG A; 3J80 c; 3BPX A; 1WJC A; 4AIK A; 2B63 J; 3U58 A; 1PTO D; 3OIQ A; 2QEJ C; 2BE5 F; 5FKK A; 1HBX G; 1U5T A; 2WB1 E; 2BHZ A; 1X3G A; 5M5W H; 1SSO A; 1LEA A; 2YU3 A; 2NVT B; 5ED4 A; 3GVA A; 5DM6 3; 3K70 C; 3B6Y A; 3CKI B; 1VQD A; 4E7Z A; 3CDH A; 1UCR A; 2EK5 A; 3H3Z A; 1IW7 C; 2JA7 H; 1VQM A; 4JV5 Q; 3BR5 A; 2V1D A; 2ZB9 A; 5B1B H; 4WWC A; 3CUO A; 1ZXT A; 2H1K A; 2VQL A; 5J1Z A; 3Q0V A; 1XMQ Q; 5CKT A; 5EPJ A; 1SAU A; 4M71 A; 1JWS D; 5K5P A; 1U9O A; 2IS2 A; 2KHJ A; 5CYU A; 4QY7 A; 4B3A A; 4O6J A; 4A3M J; 1QIL A; 1YQA A; 5H2F C; 4HF1 A; 5C3E G; 1DPU A; 1JCK B; 4Q4Z F; 2RNG A; 1FMW A; 1LB2 A; 4E7I A; 3CCV A; 3WU1 B; 4MFE A; 3FBN B; 4K8I A; 2LYC A; 2D1H A; 1HQM C; 4B48 A; 1SD5 A; 2QEN A; 3V8J A; 1S9H A; 2MW8 A; 4XT1 B; 2XIG A; 2KJB A; 2NUC A; 1E22 A; 4ENM A; 1ZKG A; 3MHB A; 2AXT C; 3F9K A; 1R5G A; 3PIO A; 4HSV A; 2B2W A; 3GZ5 A; 3G71 I; 2JA8 H; 2MH2 A; 4HMI A; 3C18 A; 4UXN A; 2ICI A; 4CDE C; 5FLV A; 3GTO J; 1J59 A; 3STB C; 5DYM A; 3VOE A; 4LK0 F; 5C0X G; 3TO6 A; 3UKG A; 3ZQ7 A; 2BVL A; 3J7A F; 2K9Q A; 1H9J A; 1H9T A; 4L6T B; 5F27 A; 2QA4 A; 1D1I A; 5FKL A; 4MNO A; 4RM3 A; 4PJJ A; 4R8I A; 3NBH A; 2R7W A; 2JA6 B; 3H92 A; 3AU7 A; 4AQY Q; 4JX4 A; 4PJN A; 2GN5 A; 3C7J A; 5HGT A; 3VPR A; 4A3L J; 3GW2 A; 1UHW A; 1QVG A; 5DNF A; 1WYD A; 3MZ5 A; 1EOV A; 3HOY J; 1R4N B; 4ILW A; 5LLQ A; 3D4R A; 4OO1 H; 2OEO A; 4BXX H; 3J7Z C; 4ZYD A; 2Q2E A; 1LV9 A; 1DUX C; 2HS5 A; 2NVY J; 3ZQC A; 4RA2 A; 3BTI A; 2QEX I; 2B63 B; 2YX7 A; 3N05 A; 1W5S A; 1JGG A; 1SLJ A; 1SJH D; 1HXY D; 2F0I A; 3LAJ A; 2LDU A; 2QBY A; 1KDA A; 3CF5 F; 3A01 A; 1IUF A; 2F1M A; 5IP7 J; 1U78 A; 5AJ3 L; 2L4N A; 1BA5 A; 3KLN A; 2ETH A; 3S2W A; 1MSG A; 1T5X D; 1LO5 D; 2ZTD A; 2D0O A; 5IP9 J; 1X3P A; 5JJI A; 1KNA A; 1RUN A; 1ERD A; 4CBV A; 1LKX A; 3ITP A; 5DCM B; 5L3E A; 1NUC A; 2P4W A; 2PPB C; 1J0R A; 2Z6B A; 1QOH A; 4AWX B; 3FPP A; 5DUK A; 4L18 B; 4LWC A; 5TGT A; 3Q22 A; 3EQL F; 2R7X A; 2P8T A; 3AL0 C; 3BQY A; 1TWF H; 2MD0 A; 1A6I A; 3OTO Q; 4XKV A; 3RZO B; 1EQT A; 3DF8 A; 2HQE A; 2H6B A; 1XMX A; 3RLN A; 2LH9 A; 3NQI A; 3W6V A; 1G3S A; 1X56 A; 3BS1 A; 1H9K A; 4BHB A; 1FGU A; 2XAJ A; 2NYX A; 1ZRD A; 4IL6 C; 3E3X A; 1TWA B; 3QOL A; 3I7F A; 3DEP A; 1GV5 A; 3H0D A; 1BDO A; 4OIN F; 1GOZ A; 5D5X B; 1Y8N B; 4BQN A; 2L02 A; 2V1X A; 2ZTE A; 3ICE A; 1MH3 A; 1RPW A; 4M9X A; 4FAS D; 1S72 A; 2QEX A; 4IUN A; 1B3A A; 1SC7 A; 3HOX J; 2ZXW H; 4N0B A; 3AL0 B; 3K5N A; 3E0J A; 2R7D A; 3HJE A; 4BGD A; 4KT6 A; 4KDP A; 1V54 H; 4DR7 L; 3C2H A; 4UVA A; 2HZD A; 1X1B A; 2R92 J; 1HF0 A; 4L5Q A; 3SXH A; 4C2M H; 2MSY A; 5E17 F; 1VF9 A; 2JA5 H; 2RA4 A; 4XRF A; 2KEC A; 4B9W A; 1WJB A; 3U1K A; 5FLM H; 3MWM A; 5FRO A; 2L5T A; 3ABL H; 4S0H B; 2DGY A; 1TP4 A; 1ZG1 A; 4DUY Q; 4NQW B; 2G7U A; 3DPT A; 2FMY A; 2K5D A; 1KDB A; 3QPS A; 1O7I A; 4EQP A; 4XN0 A; 2LXJ A; 5CJ9 A; 2O5J C; 1VQO A; 1K6O A; 1QZY A; 4KMU C; 4IAL A; 4KIF A; 5FRM A; 2QSH X; 1Z9F A; 3TZD A; 1GUG A; 1R1T A; 2HIX A; 4FVM A; 4E70 A; 2E2H H; 2QCO A; 1GHC A; 3A54 A; 3PCO B; 4CCK A; 3RSN A; 3PO2 G; 2OCC H; 1JUP A; 1XPX A; 1LQ1 A; 3L3O C; 3S2H H; 3P7J A; 2L1B A; 2IWH A; 5TJJ A; 3NEN A; 5CV8 A; 3NQO A; 3FBI B; 2FE3 A; 3P20 A; 1PH4 A; 2LP0 A; 3OYK A; 3AAF A; 2ALY B; 1Z1D A; 4NDL A; 1GXP A; 3KCC A; 3GZN B; 5C3X A; 4HV5 A; 3QJY A; 2L2O A; 4K6D A; 4HN7 A; 4KY6 A; 4C3I G; 1YJW A; 5COR A; 4BWG B; 3I4N B; 1CM9 A; 1W5T A; 2N78 A; 4W1U A; 1FSE A; 2ZJR F; 1W3S A; 4JC0 A; 4MZ9 A; 1JJ8 C; 5M3F H; 1LT4 D; 3F2C A; 2WC2 A; 4L63 B; 1Z7L A; 4D7N A; 4QIW N; 3K2Z A; 1NHA A; 1IDZ A; 1YHB A; 1SXT A; 2O0Y A; 1G3T A; 3CDL A; 2QUF A; 3L9F A; 3U9G A; 4B3T Q; 3Q23 A; 1JJG A; 4DR2 L; 2KJX A; 1SFX A; 3FMR A; 1GUO A; 4H0L C; 1FBQ A; 1BIB A; 1ZAO A; 3JTH A; 2CW8 A; 1I4H A; 1S3J A; 3RTR A; 3S9W A; 3HOX B; 2WA0 A; 3SFZ A; 2F0Q A; 4EM0 B; 1A04 A; 1PP7 U; 2PO4 A; 3PFY A; 4X3K A; 2NBF A; 4F7Z A; 2Q2U A; 2EY2 A; 1G91 A; 5G3L F; 3MZH A; 4PMC A; 3L9A X; 3PSI A; 5M3M G; 1HTL D; 3SEZ A; 4A3J G; 1V9P A; 3VYU A; 5KAI C; 4LAA A; 1ET9 A; 1U0L A; 3H0G J; 2VL6 A; 3VYR A; 2IJ0 A; 3CJQ B; 1R4Q B; 4Y7N H; 3W1G A; 3SFI A; 2Z0S A; 2CHB D; 3QR8 A; 2K5W A; 5IGG A; 2J5O A; 1DGW Y; 2MPM A; 2Q2I A; 5LGU A; 4XSZ F; 4J01 A; 1VQ8 A; 1H95 A; 4DGZ A; 1S5D D; 4BJ6 C; 4FT8 A; 2DK8 A; 5E3L A; 1IV8 A; 4P71 A; 2RHQ B; 4U0Y A; 3EYI A; 2A6E F; 1OMI A; 2G5D A; 4KHZ A; 2ZXR A; 2DUD A; 2K5N A; 1SE2 A; 3NK9 A; 2FML A; 4UV9 A; 4JX5 A; 2CWE A; 4TKO B; 3O13 A; 1IRZ A; 3QAH A; 3CW2 C; 1CQT A; 1NY4 A; 3AFQ A; 3GTQ J; 4A3I G; 4RGN A; 2VXW A; 2QWW A; 2B29 A; 5KNC A; 3CPW A; 3UE1 A; 1HNZ L; 2OZ6 A; 4LFB L; 1PYB A; 1VS0 A; 4IQJ A; 4DBQ A; 1J9I A; 5AIQ A; 4PUS A; 5F0H A; 1Y1V J; 1SR3 A; 3QB3 A; 5HDG A; 1MW7 A; 1R4P B; 5KRU A; 3LNQ A; 3CF5 A; 2B8F A; 3QWN A; 5J1R A; 3HGY A; 1STN A; 3NP8 A; 1IBK L; 2LRD A; 2ZBK A; 4IPA A; 2QDB A; 3FP9 A; 2EJR A; 4AVZ A; 2K32 A; 2UUZ A; 3HZJ A; 2ZFU A; 2GOM A; 1FP2 A; 5IY0 A; 3S16 J; 4BDY A; 3HUG A; 3OHX C; 1XX8 A; 3DLL F; 4Q5S F; 4XIG S; 5FO9 B; 4M3B A; 5E79 C; 5AJ3 Q; 5E1W A; 4LUV A; 2IXS A; 3QYF A; 4RGO S; 1NMG A; 3W3C A; 1AKH A; 3IH2 A; 3TRE A; 4K5W A; 1RC9 A; 1K8M A; 4KHV A; 3LWE A; 2Q8R E; 3BZ6 A; 4CA0 A; 1LFB A; 1G6N A; 2K04 A; 2VY3 A; 1X6O A; 1QZZ A; 1F9N A; 1H9S A; 2IEK A; 2M45 A; 4Z2Y A; 4JQF A; 3E97 A; 4ZC3 A; 3LSK A; 1F2M A; 3NTH A; 4Y33 A; 2PMZ N; 3GV6 A; 2W25 A; 1ZEL A; 2PFB A; 1FIA A; 2B2U A; 3L7W A; 2D5D A; 4LFU A; 5T1G A; 3F23 A; 1D5V A; 2B2V C; 2D0P A; 3KDF A; 5HLI A; 1BCP C; 4IKF A; 5IIF A; 2F3W A; 2OZU A; 4XN3 A; 4C3H H; 4QPO A; 4QGU A; 1E1T A; 1JUS A; 1OIS A; 2A5Y B; 1CZW A; 4LF7 L; 5I9O A; 2CFO A; 1EKE A; 3MFK A; 2HVS A; 1FPQ A; 2IHF A; 3PIP 3; 2XVS A; 2B63 H; 3RLF A; 1OCZ H; 4RB1 A; 1R9T B; 3HOW B; 2F5V A; 3JRB A; 5I2X A; 3BVG A; 5JHU A; 3ULP A; 1Q12 A; 2R93 G; 2MLK A; 2EA2 A; 1OR7 C; 3A55 A; 5GPA A; 4B43 A; 3I9V 2; 2ELH A; 2GA2 A; 2E5L L; 4ZYS A; 1GDT A; 3R0A A; 2R7Z J; 5K7F A; 4DR7 Q; 2W7N A; 2I10 A; 5JOB A; 2GWR A; 1WI3 A; 3K7A B; 2X6L A; 4JVZ A; 5EEG A; 3BDO A; 1USS A; 1YLF A; 2RDP A; 2P5L C; 2XSC A; 4OGQ C; 4R4Q A; 1A36 A; 2NZO A; 1Y1V B; 3KPH A; 1I3Q H; 4BY7 J; 4Y17 A; 3F2D A; #chains in the Genus database with same CATH homology 4LG0 B; 4U88 A; 1KU7 A; 5HLH A; 4ZKB B; 4R79 A; 1TU2 B; 1B50 A; 1CTM A; 3R3O A; 2LNB A; 1SFU A; 3I3C A; 4MTE A; 4HCS A; 4KKU A; 5COY A; 1VF5 C; 1JWU D; 2IPK D; 2KQ3 A; 1HFN A; 3IKT A; 1LDJ A; 2EV5 A; 3SR1 A; 2AS5 F; 2XIW A; 3KP7 A; 4HW0 A; 3REO A; 2EOT A; 1OXT A; 1TWC B; 5FO8 B; 4X3U A; 4ZRZ A; 3J7Y W; 2VOO A; 3KP2 A; 4OU0 A; 3BDL A; 3SK6 A; 3S16 B; 5TRD A; 5JEA G; 4C1N I; 4B8X A; 4MQV A; 5I6W A; 1ICW A; 4ZKC B; 4FXD A; 1DD2 A; 1WTQ A; 1DPR A; 2MHV A; 3NWT A; 4LMQ D; 3GLX A; 5E1X A; 2RQP A; 1ZRC A; 3S2D B; 4JBA A; 1Z47 A; 2C9O A; 3FM3 A; 3RJP A; 4CIC A; 1SMY D; 1FD7 D; 2NN6 G; 1B53 A; 1LTT D; 1OR7 A; 4JK1 C; 3ABT A; 5HLG A; 1F1Z A; 2L54 A; 2JEB I; 1PRT D; 2FEZ A; 3W2D A; 4IRH A; 3HOY B; 2XWJ B; 2G9W A; 3HRM A; 3CO7 C; 1WQ2 A; 1GXD C; 4ULL A; 1HKS A; 3SDZ A; 1WI9 A; 2ISZ A; 4OIJ A; 1CFM A; 2PMZ B; 1KU3 A; 4AVP A; 1YNJ D; 1JR0 D; 4GGG A; 1A3T A; 4EGY A; 5I9P A; 2NVY B; 2DQL A; 2FU4 A; 3HG9 A; 3I91 A; 3HKZ B; 4MGR A; 1BCP F; 1FP1 D; 4KTA A; 2LFN A; 1IZ1 A; 1EEI D; 3WTU C; 1XMK A; 1ESR A; 4ZH4 F; 1EIH A; 4NHJ A; 1Y69 U; 1HTP A; 3S17 B; 2L4M A; 2J7Z A; 1O3T A; 4KFC A; 4N9T A; 3LNN A; 3ECH A; 4RFB A; 4C3J G; 3M3Y B; 3CRL C; 4ZUI A; 1ZYR D; 2QLZ A; 1GHK A; 3G3Z A; 3IWZ A; 3EDP A; 3C46 A; 4UNO A; 3EVQ A; 3SK8 A; 1IXS B; 1MKM A; 5FLX Z; 4OIR F; 1E17 A; 1OXS C; 2M9V A; 5BPI A; 4I0A A; 1P4X A; 3HTU A; 1FNW A; 3HOT A; 1EY7 A; 1Y8O B; 3GUD A; 1SMY C; 4C56 C; 4R9W A; 2DPU A; 4U0W A; 1YIO A; 2RC4 A; 2VBY A; 3KLO A; 2LF8 A; 1B2T A; 1PRT C; 2A6H D; 5C6A A; 4RWS C; 4DXF A; 1IN6 A; 2L9H A; 5CV9 A; 4O3M A; 5I2Y A; 4EJT A; 5CMD A; 5BOX A; 4NB5 A; 2CQA A; 4X3T A; 3JSO A; 1F2Y A; 1YNJ C; 3TGN A; 5FLX L; 5LHY 1; 2F0U A; 4HF2 A; 2CW0 D; 3NXW A; 3K69 A; 2ZT9 C; 2D1V A; 1WTO A; 4PGH A; 3QP5 A; 4OO1 G; 2O3F A; 3NEU A; 4KMU X; 4EVO A; 5JPN C; 2HYG D; 4KV6 A; 1HST A; 4A0Y A; 2OT2 A; 1TQM A; 1KAA A; 1ET6 A; 1ZRE A; 5DDG A; 1I4P A; 2EY1 A; 1J5Y A; 1ZRF A; 4KNY A; 1ZYR C; 3VR2 A; 1T98 A; 1YTY A; 5JI6 A; 1SAP A; 5FLX E; 1CGP A; 3RUZ A; 4IFD H; 1ZHF A; 2EZV A; 5ILS A; 1SNP A; 4RGR A; 3K1P A; 5HDK A; 2O61 A; 4F7X A; 2QM3 A; 1BI1 A; 2LF7 A; 4FHT A; 3PFI A; 2DTR A; 1JC7 A; 4HAM A; 1PMR A; 3VR5 A; 3Q9V A; 1LT6 D; 4J6H A; 2JE6 I; 1NCV A; 1AW7 A; 1ZGA A; 3D0S A; 1QO0 D; 1B9N A; 2A6H C; 2AQ1 B; 5IGB A; 1I5Z A; 2D62 A; 4UHT A; 2IW5 A; 3JAM Z; 1TS5 A; 3KZZ A; 1XCB A; 2EWN A; 2RQX A; 5BMZ A; 4XBF A; 4LJZ C; 2WKC A; 1V1P A; 1OYW A; 2CW0 C; 1O7F A; 2Z99 A; 1V1P B; 5EPL A; 4LLN A; 2ADU A; 3MA2 B; 4L8J A; 1EQV A; 4PNY A; 3BY6 A; 4CKC A; 4ZZL A; 5KEE A; 3D8G A; 1OXU A; 5F2D A; 5HS7 A; 3K57 A; 3ZYY X; 2BOS A; 2F0K A; 5DEQ A; 4KYW A; 1P4A A; 3NTI A; 3NTK A; 5M5W B; 3TQN A; 4XYQ A; 3G73 A; 2R7Z B; 3JAM L; 3TSS A; 1QNT A; 5IOD A; 2Z5U A; 2Z33 A; 3L7Z C; 2R93 B; 2EB7 A; 2JA7 B; 1W6K A; 3P9K A; 4N9H A; 1D1K A; 2VT3 A; 3JAM E; 1TT2 A; 3F8B A; 1YO5 C; 4XDX A; 5JR3 A; 4BBR B; 1BQQ T; 2GQQ A; 1CT1 D; 1SDF A; 2Z4H A; 4P9U A; 5LHG A; 4MEX F; 4UUV A; 4LLL A; 2PLY A; 2QCP X; 5D5U B; 2A07 F; 1QWY A; 2K28 A; 1STH A; 1IN5 A; 3IKJ A; 2FBH A; 3J80 K; 2HKX A; 2KCN A; 4CHU A; 1S7A A; 4KY7 A; 2JPB A; 2BGC A; 1R58 A; 1TBX A; 4BXU A; 1L0O C; 4R8H A; 4MIU A; 1A3V A; 3RD4 A; 2QQA A; 1RF2 D; 2K27 A; 2EYM A; 4KK7 A; 3ECO A; 3URY A; 3I90 A; 1EH6 A; 5IGF A; 2STT A; 3WTV C; 3V4G A; 1D6E C; 5K5R A; 5CC4 A; 2AQ2 B; 4HCE A; 1VMP A; 4LK1 C; 3ZMV A; 3HS0 C; 2EFQ A; 3R4K A; 1BXT A; 3GZ6 A; 1EU4 A; 2B8G A; 4UDU C; 2CFX A; 3ZMU A; 2E74 C; 2DAO A; 1IYV A; 2F0F A; 1R36 A; 5TSS A; 4BNC A; 1RNL A; 5KNB A; 1SMY F; 2R92 B; 4QLC U; 2EYO A; 1RCV D; 3CTA A; 2IA0 A; 3FRP B; 3SE0 A; 1PRT F; 5HS9 A; 3HOU B; 2VOD A; 1MSH A; 2KUM A; 4F87 A; 1PLF A; 2EFW A; 2K33 A; 3V8K A; 3GTP B; 1CHP D; 1C4Q A; 4HTH A; 2HQR A; 1BOA A; 2PJP A; 5LRS A; 3K59 A; 2K6A A; 3Q5F A; 5JVG 3; 2A73 B; 2F0V A; 2FOK A; 2MUY A; 1G6Z A; 5ELE A; 1BOS A; 3WG9 A; 1UHM A; 2HGC A; 4EJW A; 4OL7 A; 3WTT C; 1SFE A; 2FNP A; 2F3V A; 4OIQ F; 4ON0 A; 3K6O A; 2NNN A; 3DMU A; 4N9P A; 4G57 A; 4YFK F; 1ZYR F; 2D2C C; 1YS7 A; 4WK8 F; 4ABN A; 2MP1 A; 1ZN2 A; 1RZ4 A; 1CHQ D; 2E2D C; 1FBU A; 1Z05 A; 2PW7 A; 1BJA A; 5FO5 A; 3VR4 A; 1WD1 A; 3E6B A; 3ERE D; 2F5F A; 3KET A; 3CU7 A; 4AYB B; 2A6H F; 2EFN A; 1BUV T; 4WF2 A; 4ESJ A; 2BYV E; 4IJA A; 2F0P A; 2XRS D; 4BY1 B; 1R22 A; 4C3J B; 4DNC A; 4LJZ F; 4OMY A; 4OMZ A; 5FB2 A; 1M4V A; 4KD4 A; 3BWG A; 1QBJ A; 4V0B A; 5CBE E; 2CW0 F; 1E2X A; 3GQB A; 5DD8 A; 1O3R A; 4K2E A; 4U7D A; 2DNE A; 2PI0 A; 3BYY B; 1XCV A; 5A35 A; 4RB2 C; 2V6C A; 3BP8 A; 2IX1 A; 5ICC A; 1JE4 A; 2RGV A; 4E6I A; 2TSS A; 1SYG A; 2NTS A; 4M73 A; 1EOT A; 3J7A V; 5IGE A; 3WU0 A; 4C1N K; 1O7Y A; 1XTC D; 3CJN A; 1WD0 A; 5ELC A; 3F8C A; 5JEA H; 4FX0 A; 3GTL B; 1YW7 A; 2X69 A; 1Z91 A; 4XSX F; 2KVM A; 5K36 G; 3GWZ A; 3J81 Z; 3DWA A; 3P1H A; 4FP5 D; 3V8L A; 4CZZ A; 1ENC A; 1JGS A; 3KP3 A; 3BYT B; 3S1M B; 1KU9 A; 4G94 A; 2NN6 H; 4CGY B; 1MJB A; 3ISP A; 4FX4 A; 3RZD B; 1PDN C; 1Z9C A; 5D4S A; 4RKL A; 4MN3 A; 3UI2 A; 3WGG A; 3I64 A; 1R23 A; 1HPC A; 3T5X A; 3OIP A; 4UV8 A; 1HQC A; 2C6Y A; 3KP5 A; 2JZY A; 1MBY A; 1JHF A; 3BRO A; 1IN7 A; 1OPC A; 3A8I E; 2H09 A; 2ZXP A; 2MA3 A; 3DWQ A; 3F6O A; 3J81 L; 2GAU A; 3M9A A; 1S7O A; 5D8K B; 5JAV A; 1V1O A; 4AD9 A; 2O5I C; 4L9T A; 4DK1 A; 4NDX A; 4WRD A; 3ROU A; 2DLL A; 4EVI A; 4XLN F; 3FEW X; 2H94 A; 4M72 A; 4G8X A; 3HOZ B; 3D5L A; 5DUI A; 3TP8 A; 3T53 B; 5KYI A; 2YPR A; 3QP6 A; 1FNU A; 4JBW A; 2M8E A; 2J5P A; 2VNU D; 3J81 E; 1JE8 A; 3S1N B; 4FF2 A; 5C0X J; 1QG7 A; 1STY A; 4LK1 F; 5KVR A; 2WB1 B; 1HQR D; 4P2C B; 3D6W A; 1JIC A; 2F0D A; 2DW4 A; 2ZCW A; 2HCI A; 4CJ0 B; 2OA4 A; 4TRD A; 4I02 A; 3MXN A; 1KGS A; 4U67 3; 1H9M A; 2YSR A; 5D6F A; 2NVX B; 2QF7 A; 4G7Z C; 2W57 A; 3SHF A; 3H09 A; 2XAS A; 4HSP A; 3Q9S A; 2NAZ A; 3D3R A; 3MXP A; 1T39 A; 2NB0 A; 1LTI D; 2B2T C; 5BQT A; 3X3N A; 2KA7 A; 4HLY A; 1P2F A; 2BEO A; 1O7L A; 4A93 B; 2ZXO A; 4I0B A; 5FLX K; 1Z6R A; 4HOB A; 5HUR A; 5EGT A; 3GFJ A; 1U4L A; 2G9H D; 2EYL A; 4O1N A; 2NRA C; 4NKL A; 4RKB A; 4WJW A; 5KYL A; 3F8N A; 1RIO H; 4K8J A; 1BI0 A; 4BYY A; 2A69 D; 2IPQ X; 1Y8P B; 1GUW A; 1LVA A; 4ODG A; 5EQ0 A; 3RYR A; 3T13 A; 2BBY A; 2PNR C; 5E8I A; 3PUW A; 2FXA A; 4MHG A; 3QG1 A; 1O57 A; 1E2V A; 4IUT A; 4IHT A; 1XMA A; 2XKO A; 5FO7 B; 4CHT B; 2L12 A; 1STB A; 3BDD A; 3L2C A; 3TKY A; 1W7P D; 4G7H C; 2BA1 A; 3H0G B; 1D3Y A; 1JB3 A; 5J22 A; 4IFD I; 2H6C A; 1IXC A; 1DM0 B; 4HMJ A; 4DAV A; 3M7N A; 1G3Y A; 2RDG A; 3CHB D; 5CV6 A; 1CF7 A; 3GP7 A; 1XGO A; 5E3F A; 1CF7 B; 3TP5 A; 4YFN F; 1V4R A; 1PFN A; 2BV6 A; 1WSU A; 4KUM A; 1TLH B; 1FYL A; 2QQ9 A; 4M1U B; 3C1E A; 3NHH A; 1E2W A; 1TWG B; 5IT9 K; 4A0Z A; 1ZAR A; 1IKL A; 4MNU A; 3TBI B; 3K5L A; 4EGV A; 4IF4 A; 1TQP A; 1MJ9 A; 4EQN A; 4KN7 C; 4BA1 I; 2Z9O A; 4LB6 B; 1IAW A; 1S5E D; 2ISY A; 2JPC A; 5JPM C; 1VF7 A; 2OAZ A; 4IRG A; 1FYM A; 1U4M A; 3HRT A; 2A69 C; 1ODD A; 3CUQ A; 1RDP D; 3FHZ A; 3JAM K; 3HOV B; 3CUQ B; 1WTP A; 1DP7 P; 1I4Q A; 3I71 A; 4IQZ A; 1C8C A; 2B2T A; 1TQO A; 2F0S A; 1TY2 A; 5CVI A; 1I4X A; 5DM7 3; 5CGK A; 1A2U A; 2VE9 A; 5LGN A; 3SK5 A; 4ASN A; 2F0W A; 1U8R A; 1YW9 A; 1SNC A; 2Q0O A; 1DYQ A; 4EV0 A; 4I7H A; 4GYI A; 3PQK A; 3ZP5 A; 4LB5 A; 3Q24 A; 3OV8 A; 2VXZ A; 2D45 A; 3TN2 A; 1QGP A; 3LWF A; 3E6Z X; 4RB0 A; 1P6R A; 1SE3 A; 2KIU A; 3BJO A; 1ZG3 A; 3GTQ B; 2C5C A; 2X4H A; 4KN4 C; 2KED A; 1D2B A; 3TOA A; 1Q8I A; 3ONA B; 5DV5 A; 3FMC A; 3OWF A; 3KBG A; 4RS8 A; 1CQF A; 3A8K E; 4PMB A; 3K5M A; 2V79 A; 1XXG A; 1M8A A; 1FNV A; 2B2V A; 2PZU A; 5ICE A; 3U9S A; 1ULY A; 1CA5 A; 5F6F A; 2JA5 B; 4M74 A; 2EFO A; 2AWO A; 1C48 A; 5A2Q Z; 3T56 B; 1VDZ A; 3LQR A; 1BCP B; 4MEY F; 5L3C A; 1O7Z A; 1HA5 A; 4KKT A; 1DOK A; 1KA8 A; 2NZ1 D; 1MD0 A; 1KYW A; 1JQY D; 2WP8 J; 4BA2 I; 1NAP A; 2XGX A; 2FIN B; 3E5U A; 1FBS A; 2WV6 D; 2E2I B; 4A0X A; 4KA4 A; 1I6X A; 2UWM A; 2CO5 A; 4PCQ A; 4G7Z F; 3OWE B; 2BCO A; 1KYZ A; 3HYI A; 2OXP A; 2Q8K A; 5A2Q L; 3FKI B; 1HA6 A; 4KIG A; 3FMS A; 2P6T A; 3E6D A; 1EYD A; 1K83 B; 1MDM A; 1ZLK A; 1Y1W B; 3U1D A; 5F64 A; 1X32 A; 1HKT A; 2QQB A; 1FT9 A; 1MDM B; 5C4Z A; 4RAY A; 2VB2 X; 1SD4 A; 2MBF A; 3FM5 A; 1LDK B; 1W7P A; 5LRR A; 5ISR A; 3LA2 A; 5IGD A; 1FC3 A; 2K4B A; 1W7P B; 2VC1 A; 2SOB A; 2E2J B; 1ENA A; 3C3L A; 3F72 A; 3NBI A; 5I4D A; 5A2Q E; 1PDQ A; 4M9S A; 1KAB A; 1S6L A; 5LHH A; 1FLI A; 3LAP A; 2EJF C; 3MQ0 A; 1FYK A; 5L3B A; 2ENB A; 1XYI A; 2JEA I; 2FBI A; 1IYU A; 2HDC A; 4F88 A; 5CVR A; 3V7R A; 3ABU A; 1O80 A; 1O78 A; 4DK0 A; 2AXL A; 4G7H F; 1F9Q A; 4G6Q A; 4I01 A; 3D4W A; 3C1D A; 4A6D A; 3ZPL A; 4NMZ A; 2M87 A; 5LL6 S; 4Q0F A; 4YIF A; 2P6S A; 3EFX D; 3DXJ C; 3F6V A; 1AP0 A; 4EQQ A; 3ZMT A; 1E0B A; 2FHT A; 1ML0 D; 1A2T A; 1K7A A; 3CLO A; 4KD3 A; 3WOF A; 4FXG C; 2P5K A; 1RTN A; 1IXR C; 1TVX A; 2H27 A; 3T4A C; 2GIV A; 4GZY C; 3GX4 X; 3NSW A; 1LDD A; 5BN5 A; 1U6G A; 3C1F A; 3MZY A; 5IT3 A; 2FSW A; 3NWS A; 6PAX A; 1U9R A; 5FD5 A; 2FF4 A; 5D6E A; 1TY0 A; 1EWH A; 4NQW A; 2K7V A; 2FFK B; 2L11 A; 2HCC A; 4ETS A; 5K5Q A; 3LA3 A; 1R5H A; 2A69 F; 4A5N A; 1LAB A; 2BE5 C; 3TP7 A; 1HXD A; 3SEB A; 1F4K A; 4S04 A; 1ILQ A; 1IHZ A; 2SDF A; 4IUR A; 1S5C D; 5ICG A; 1IN8 A; 2E7W A; 1HUN A; 2P7C B; 4H44 C; 2ZME A; 1CQV A; 3MAQ A; 1TS3 A; 1EIG A; 5M3F B; 2YR2 A; 2ZME B; 1ONV A; 2G7H A; 4A3B B; 5BS6 A; 5KK1 A; 3PV0 A; 2VT2 A; 3RKY A; 2VON A; 3H6M A; 3PQJ A; 3QPH A; 2AQ3 B; 3FXR A; 1XWE A; 2O2L D; 2XNA C; 2NTT A; 1LTG D; 4DA2 A; 3DFG A; 4BH9 A; 1Y0U A; 1SE4 A; 3LST A; 2HYF A; 2NN6 I; 2W84 A; 5DD4 A; 2E7X A; 1EH8 A; 1W6J A; 3H94 A; 4XT3 B; 4GYG A; 1Q3L A; 4I98 B; 1Q90 A; 1D8J A; 2OF1 A; 3E3V A; 3M8E A; 5FD6 A; 2DK5 A; 4M8A A; 1UNS A; 2I07 B; 3VR6 A; 3F21 A; 2P7V B; 1KQ8 A; 1GUS A; 2VUM B; 3GFI A; 1HCZ A; 4RAU C; 4LD5 A; 2K4J A; 3DEO A; 1FR3 A; 1I27 A; 3ND9 A; 3OMG A; 2H4O A; 3CO6 C; 2NYZ D; 3I4P A; 3RPQ A; 4NP5 A; 4HF0 A; 3WOD F; 4HX4 A; 5F1R A; 3DPL C; 3E6M A; 3BG3 A; 3CRK C; 5ERI A; 3I4L A; 1U2W A; 4H0E A; 5LHI A; 4KT5 C; 3KLS A; 3ND8 A; 5CBA E; 4XQN A; 2F5D A; 2CSO A; 3BVZ A; 3ERQ A; 1STZ A; 5E18 F; 1R00 A; 3DKW A; 1QE6 A; 1DJR D; 1U5T C; 1BI2 A; 2DFI A; 1O3S A; 4P55 A; 3SXY A; 2Z4I A; 4BHC A; 4WOR A; 1QCB D; 3HHH A; 1MD2 D; 1PRT B; 1J2X A; 4WFN 3; 1TS2 A; 3R6S A; 1PUE E; 2EV6 A; 4A12 A; 4Q5V A; 1TNT A; 3OOP A; 4PDK A; 2WV0 A; 1UFM A; 1I4G A; 4QUC A; 4HYE A; 2O8X A; 1Z7T A; 3VFZ A; 2DT5 A; 1HSJ A; 1YNN D; 4OO1 I; 2J4X A; 4L5I A; 4UVB A; 3KLR A; 3B02 A; 2HTJ A; 1BM9 A; 3NRV A; 1T38 A; 2HZT A; 1IKM A; 4BY7 B; 3HRZ C; 1EY4 A; 2F5C A; 4ME5 A; 3E5S A; 3ML6 A; 1LT5 D; 4ZAI A; 1J7K A; 4K2K A; 2EV0 A; 1QPM A; 1TW3 A; 3G6J B; 2F4I A; 4N9I A; 2DN8 A; 2RN7 A; 4DQ2 A; 2Y0M A; 3DXJ F; 3VR3 A; 4MQ9 F; 1F9P A; 1IW7 D; 5CV7 A; 3QOP A; 3KEO A; 4RH6 A; 1LTR D; 3GXO A; 4AIH A; 2OBP A; 3W6J B; 1AOY A; 3U9O A; 2QIL A; 2D2W A; 3BPX A; 4AIK A; 3A56 A; 3I58 A; 2Z3Y A; 1RI7 A; 3WGI A; 4CJ2 C; 1PTO D; 3CQZ B; 2ZNY A; 3OIQ A; 4M9Z A; 5LBQ A; 3MFY A; 4ZK9 B; 1HW5 A; 2QEJ C; 2K1B A; 2BE5 F; 1YNN C; 3I72 A; 4HED A; 4ID6 A; 2NS0 A; 2XAH A; 3I59 A; 1CZG A; 2B0L A; 1HBX G; 2KKO A; 2RDF A; 2RSO A; 3I59 B; 4RB3 C; 2LSO A; 3IHU A; 3DWP A; 4PMW A; 1U5T A; 5JVT A; 1XSD A; 1U5T B; 1JOK A; 1SSO A; 1D5M C; 1LEA A; 2YU3 A; 4WXD A; 4O42 A; 2NVT B; 2MJ8 A; 4YIJ A; 2ACJ A; 2F0L A; 4NW2 A; 1Q1H A; 2XWB B; 3P9I A; 5ED4 A; 3GVA A; 5DM6 3; 1TTY A; 2P5V A; 4ESF A; 4I2O A; 3CKI B; 1SYD A; 5E1Z B; 1GXQ A; 1P92 A; 4HQM A; 3V05 A; 1BIA A; 4A6E A; 2B2W C; 4CKB A; 3CDH A; 1UCR A; 4Q47 A; 2JA8 B; 2EK5 A; 1MGS A; 1EU3 A; 3H3Z A; 3GTM B; 1IW7 C; 2RKH A; 4PZJ A; 1OYI A; 3K1M A; 1IUY A; 2E76 C; 1ILP A; 4EJU A; 2V1D A; 4Y13 A; 1B6A A; 3P7N A; 4WWC A; 3CUO A; 3AMU A; 1ZXT A; 2I46 A; 1HW2 A; 1EY6 A; 5J1Z A; 1PTO C; 3GTG B; 3NUC A; 4YII A; 5EPJ A; 2W24 A; 1QJO A; 1JWS D; 4M71 A; 2L4A A; 5K5P A; 5M9O A; 4EGZ A; 2IA2 A; 5CYV A; 5CYU A; 1SYE A; 4L0Y B; 5JVH 3; 1WWX A; 2NNY A; 2V4D A; 2F2E A; 4XXE A; 2FJ2 A; 4O6J A; 1S29 A; 2HDL A; 4CO8 A; 1QIL A; 3WTY C; 4EQO A; 1YQA A; 2ZJ2 A; 1KLU D; 3R0J A; 1G2S A; 2A74 C; 3V7S A; 3I53 A; 4HF1 A; 1DPU A; 1JCK B; 3I5U A; 4Q4Z F; 2L55 A; 1HKQ A; 2IA1 A; 2L01 A; 3RKX A; 1LB2 A; 3WU1 B; 5JFR A; 1EL0 A; 4M6Y A; 4WX9 A; 2K7L A; 1TKW B; 4RHS A; 4M6X A; 3IAY A; 2Z1C A; 2Y48 A; 3KK7 A; 5HS8 A; 4K8I A; 3NQT A; 1SBB B; 2D1H A; 1HQM C; 1SD5 A; 3OPO A; 5FKA C; 2QEN A; 3FVQ A; 1XB4 A; 3M7G A; 3V8J A; 4A3K B; 5A2Q K; 4IUP A; 4DN4 M; 2QKI C; 1HRJ A; 3ZMS A; 4XT1 B; 2XIG A; 1OKR A; 2KJB A; 2JSC A; 2NUC A; 3HP3 A; 4KIC A; 1FWZ A; 4ENM A; 5FK9 C; 3MHB A; 4I09 A; 3H91 A; 1AZQ A; 3HGB A; 4BBS B; 1R5G A; 2XVC A; 2F03 A; 2PQ8 A; 2KEE A; 2K86 A; 2V9V A; 4HSV A; 5EBC A; 3KF8 A; 3X3M A; 3K9T A; 4G4K A; 2B2W A; 3GZ5 A; 1RTO A; 3S14 B; 2MH2 A; 4HMI A; 3C18 A; 4UXN A; 5K1Y A; 2ICI A; 2R61 A; 1EZ6 A; 3EQL C; 5E1F A; 3QO2 A; 1J59 A; 5CVJ A; 1XDS A; 3PO3 B; 1KDC A; 3GYH X; 5DYM A; 3VOE A; 1XGN A; 3HIF A; 4LK0 F; 5EPK A; 5C0X G; 3TO6 A; 5I6Y A; 1PTO B; 2JYO A; 3PUY A; 3ZQ7 A; 1STA A; 1TW2 A; 3J7A F; 3I8Z A; 2BDN A; 1H9J A; 1H9T A; 4L6T B; 5DAU A; 4DF7 A; 2OQG A; 2KIM A; 4LMY A; 1PZK D; 5ELF A; 1CK1 A; 1D1I A; 3D6C A; 3ZN1 A; 5H20 A; 5EKK A; 3P75 A; 1I6V C; 2JSO A; 3FXQ A; 3BZD B; 4YG2 F; 1DDN A; 4K5X A; 4R8I A; 2XAQ A; 2X6G A; 4FF1 A; 1PFM A; 2B2U C; 2R6G A; 3NBH A; 1FZP B; 2JA6 B; 3H92 A; 2M00 A; 2DBB A; 3AU7 A; 3LWU A; 4X3S A; 4DNT B; 5E8G A; 1E2Z A; 1HW1 A; 5C5M A; 3T8R A; 2IX0 A; 3C7J A; 2VBW A; 5HGT A; 2HR3 A; 1G3W A; 4DU9 A; 3GW2 A; 2Q8I B; 1W4M A; 3I54 A; 4H13 C; 1WCM B; 2K05 A; 1UHW A; 4RGX A; 2O4X A; 5DNF A; 1D5Z C; 5C4J B; 3MZ5 A; 2CGP A; 1DXM A; 4EM1 A; 1GHJ A; 1SNM A; 1XSX A; 3JSP A; 1VCI A; 2DO7 A; 3WTW C; 4ILW A; 2LS0 1; 4OIK A; 5JJZ A; 3MC0 B; 5BN4 A; 5LLQ A; 4IFD J; 2VE8 A; 4PQX A; 3D4R A; 4OO1 H; 2OEO A; 1IW7 F; 3OMC A; 1BBX C; 1CA6 A; 4ZYD A; 4QWQ A; 1ENF A; 5NUC A; 2Q2E A; 1LV9 A; 2M1B A; 2UXX A; 4IRI A; 1DUX C; 3KBX A; 1TWF B; 2HS5 A; 4B9X A; 2B63 B; 3RDI A; 1T6S A; 5C4X B; 2YX7 A; 2MGS A; 1W5S A; 3M4O B; 1PTO F; 1SJH D; 1HXY D; 3IC7 A; 2AUK A; 2F0I A; 4U7B A; 2WII B; 4KY5 A; 3LAJ A; 4WXH A; 3E5X A; 2LDU A; 3RIR A; 4RGS A; 2QBY A; 3HSR A; 3ZMD A; 2QBY B; 2HOE A; 1KDA A; 1IRF A; 4XLP F; 1PXU A; 2PMU A; 3GTK B; 2XHK A; 5FGM A; 1FNN A; 2EYH A; 2L0C A; 2F1M A; 1U78 A; 1TQI A; 2PBI A; 2SNM A; 2AWN A; 5LL6 X; 1V3F A; 4I65 A; 2L4N A; 3TDZ C; 3KLN A; 3EJI A; 2G9D A; 2ETH A; 2LKV A; 5HDN A; 2PW5 A; 2MD5 A; 1AN8 A; 3S2W A; 1MSG A; 1T5X D; 2BA0 A; 3FXU A; 1LO5 D; 2EPB A; 3D31 A; 2CVR A; 5ICF A; 3N4M A; 3DM1 A; 4DFA A; 1SD6 A; 1X3P A; 2F0G A; 2RVC A; 1KNA A; 1RUN A; 3M85 A; 4CBV A; 3IL8 A; 1U4R A; 4HR7 B; 2L3D A; 5DCM A; 5E4X A; 3ITP A; 3EET A; 5DCM B; 5L3E A; 3Q6S A; 2V1U A; 4DXG A; 1NUC A; 2P4W A; 5AFW A; 5EKI A; 1QNK A; 2PPB C; 1C0W A; 1J0R A; 1EWC A; 1QOH A; 4AWX B; 3IRR A; 4GXO A; 1Z6H A; 1SYB A; 5ELD A; 3FPP A; 1CI3 M; 1OO9 B; 3MWY W; 3JW4 A; 1KNE A; 5DUK A; 2IX3 A; 3I73 A; 1YS6 A; 3L09 A; 1G29 1; 4L18 B; 2KHS A; 2FMC A; 2IP2 A; 1BR9 A; 3AB9 A; 1DON A; 4Z9C B; 3Q0A A; 1SYF A; 1I1G A; 3IL2 A; 3Q22 A; 2COM A; 2GA4 B; 1WTW A; 3EQL F; 4RCN A; 5D8L B; 2P8T A; 3SZP A; 1Z7U A; 1WKM A; 3G7L A; 1B9M A; 2A5H A; 1OXV A; 5HVQ C; 1GJX A; 2XKP A; 1REP C; 3RZO B; 2HQN A; 3R60 A; 4LGW A; 3OW7 A; 3HHG A; 4K14 A; 3P9C A; 4ENN A; 1LEB A; 1MZB A; 1EQT A; 3IKV A; 3EA6 A; 4HDU A; 2VOP A; 4S05 A; 2JTV A; 3DF8 A; 1LTA D; 1AWC A; 2HQE A; 2H6B A; 2EE1 A; 2IRF G; 4CJ1 B; 4C2M B; 2ICE C; 2EVB A; 3NQI A; 3R8B A; 4ZYE A; 4OOI A; 5DEH A; 1G3S A; 5C3E B; 3BS1 A; 1H9K A; 1II3 A; 4BHB A; 3WTS C; 3BPV A; 2MC3 A; 2XAJ A; 2XUB A; 4HV6 A; 2WWY A; 3F2U A; 2NYX A; 1ZRD A; 1I4R A; 1JOO A; 1LAC A; 2VB3 X; 2EY5 A; 3FWE A; 1TWA B; 3QOL A; 3DEP A; 1AEX A; 4KMF A; 1F2L A; 1BC8 C; 2E75 C; 1SYC A; 5D14 A; 1S8N A; 3RYP A; 1HUM A; 4EJO A; 1G4D A; 1BDO A; 1RJT A; 2N55 A; 1TNS A; 4J1M A; 4KJN A; 5EBD A; 3VA5 A; 1D8K A; 4OIN F; 2WAQ B; 2X0L A; 4IVN A; 5D5X B; 1FOK A; 3RKW A; 1GOZ A; 3QOJ A; 1Y8N B; 3QU3 A; 2F0T A; 4R1H A; 4QF4 A; 2ZO4 A; 2YU9 B; 1IRG A; 2KJC A; 2L02 A; 2V1X A; 1UUP A; 3SHL A; 4O5V A; 3VYS A; 1UB9 A; 5CVV A; 1EYC A; 4A3F B; 1LJ9 A; 1P4W A; 5C5L A; 4G7O C; 4AIJ A; 1ON2 A; 4QUF A; 1U3E M; 4DAP A; 3U2R A; 5J8C A; 2EYP A; 4M9X A; 1GUT A; 4FF3 A; 2CQK A; 4ZZD A; 1ZKO A; 4IUN A; 3DEU A; 2FMM A; 2MH9 A; 5C44 B; 1B3A A; 3LQQ A; 4ENK A; 3RMH A; 2PMH A; 4OIO F; 2GXG A; 4N0B A; 5CV4 A; 1WTR A; 3S1Q B; 2BE5 D; 1H9G A; 4RGU A; 3ERO A; 2JXM B; 3T16 A; 5DCF A; 3K5N A; 3E0J A; 2R7D A; 2E2H B; 3DUZ A; 5ILV A; 1EYA A; 1R1U A; 4I7Z C; 4KDP A; 3DP7 A; 5G3L D; 4FF4 A; 3BVM A; 3B73 A; 4UVA A; 1XGM A; 1B1B A; 5L3D A; 1FYC A; 4K2L A; 3KZY A; 5K5O A; 1TII D; 3VEP A; 1EFI D; 3S2H B; 2PQE A; 4UAI A; 2A3S A; 1ON1 A; 3SXH A; 2RNJ A; 1QQI A; 4DNR B; 5E17 F; 2H1E A; 4KKS A; 1WVL A; 3ELK A; 2RA4 A; 2FNA A; 4LLG F; 4XRF A; 2JP1 A; 4JK1 X; 3FMQ A; 3HOS A; 4HX8 A; 2KEC A; 4B9W A; 2O03 A; 2HEO A; 3MWM A; 1Z6T A; 3S15 B; 2HWV A; 5JEA J; 4OHJ A; 1TS4 A; 2L5T A; 3EUH A; 2WKD A; 5D5V B; 1ZG1 A; 1MI2 A; 4K5V A; 3TDU C; 1BBY A; 2PZW A; 1F2Z A; 4L5J A; 1ZG5 A; 2G7U A; 1L3L A; 3I0T A; 4PV1 C; 2AUJ D; 3ZMZ A; 3DPT A; 2FMY A; 2IVM A; 1MJA A; 1A15 A; 2K5D A; 2TMP A; 1KDB A; 4J8Q A; 2ZXJ A; 4EQP A; 5K36 H; 2VBZ A; 1WTV A; 2WTE A; 3GFL A; 1LTS D; 5CJ9 A; 2O5J C; 4IUQ A; 2FRH A; 3HEJ A; 3BDC A; 1K6O A; 4U0V A; 1BC7 C; 2A6E D; 3KLS X; 1RUO A; 1QZY A; 2TDX A; 1KLG D; 2EFP A; 4KMU C; 1S5B D; 4IAL A; 4KIF A; 4TSS A; 3GO5 A; 3HSE A; 1TWH B; 4TV7 A; 3W6K B; 3PMF A; 3BJ6 A; 3TZD A; 1X3U A; 2GA1 A; 1GUG A; 2SNS A; 1R1T A; 4FVM A; 5C0X H; 3V96 A; 1FSH A; 4E70 A; 3MXG A; 5CVU A; 2K01 A; 1GHC A; 4D0P A; 2R3Z A; 3A54 A; 1ZLJ A; 3J81 K; 4GO1 A; 5KND A; 1B59 A; 2KCC A; 1L0Y B; 4G6D A; 2WIN B; 1J75 A; 3KP4 A; 1LQ1 A; 3L3O C; 4BS9 A; 1BF4 A; 5AON A; 3MAJ A; 4MTD A; 5LGT A; 2O6G E; 4B09 A; 3P7J A; 1QNU A; 2F0M A; 2L1B A; 4L9N A; 1F9S A; 2IWH A; 3HSF A; 2EDG A; 3LX0 A; 5TJJ A; 5CV8 A; 4A3D B; 3NQO A; 3FPU B; 4HX7 A; 1EY8 A; 2FE3 A; 2UZK A; 3P20 A; 2A68 D; 1XN7 A; 3MTS A; 2JT1 A; 2WAC A; 3S1R B; 3AAF A; 1BO0 A; 5IT9 Z; 2A6E C; 4P5O A; 3O6B B; 2R7F A; 3BOQ A; 1Z1D A; 1GXP A; 3KCC A; 5C3X A; 4HV5 A; 3QJY A; 4K6D A; 1I1S A; 4HN7 A; 3TO9 A; 1R1V A; 4KY6 A; 4G9Y A; 4C3I G; 3OSO A; 1DOL A; 3PUV A; 5COR A; 5KGU A; 3K58 A; 2ESH A; 1S4Z A; 4BWG B; 3I4N B; 1CM9 A; 1W5T A; 4A5M A; 2HTS A; 1FSE A; 1J8I A; 2F5E A; 1K78 A; 3M8F A; 4G7O F; 4RGM A; 5M5X B; 4P9F A; 1K78 B; 2Y75 A; 4IUU A; 1FGB D; 2PG4 A; 2UXN A; 3IRQ A; 1LT3 D; 2DPD A; 5IT9 L; 4C3H G; 1D5X C; 3V7C A; 2RDH A; 2PZT A; 1LT4 D; 2WC2 A; 2B2Y C; 1J9O A; 4L63 B; 3KEQ A; 4HA8 A; 3WOE A; 4CYD A; 3VOD A; 4Y7N B; 1UAP A; 3K5O A; 1F5T A; 1STG A; 4XYO A; 1PFB A; 2YYZ A; 1TR5 A; 3TP6 A; 3K2Z A; 1NHA A; 2EYJ A; 2A68 C; 5IT9 E; 1BN5 A; 1G8Z D; 3ULQ B; 1OYY A; 5L3G A; 4QCL A; 1SNQ A; 1SXT A; 1LNW A; 2O0Y A; 2F0H A; 2KIF A; 1G3T A; 4IZ8 A; 5D65 A; 3SZT A; 1Q1B A; 2QUF A; 3L9F A; 3E6C C; 3FF5 A; 2OD5 A; 2OU2 A; 3U9G A; 1KQ9 A; 1RKN A; 2W85 A; 1IN4 A; 2XRQ D; 5EJW A; 3Q23 A; 2KOL A; 3C3W A; 1NR2 A; 1A3U A; 2KJX A; 1SFX A; 3FMR A; 1GUO A; 2FBK A; 3MFG A; 1K8O A; 4H0L C; 1FBQ A; 1BIB A; 1ZAO A; 3RPU A; 1U4P A; 3HZX A; 2D9U A; 1EZ8 A; 5D5W B; 4ZQ3 A; 1EY9 A; 3GF2 A; 3JTH A; 3IWF A; 3GTJ B; 1I4H A; 2IL8 A; 1S3J A; 1YYV A; 1SND A; 1VMC A; 3RTR A; 3S9W A; 3HOX B; 5FFZ A; 3SFZ A; 2F0Q A; 4CDG A; 4EM0 B; 1A04 A; 2ZFW A; 1PP7 U; 2E1C A; 2PO4 A; 3NE5 B; 4MHE A; 4X3K A; 2NBF A; 4FXK C; 1JXS A; 2CYY A; 4F7Z A; 2SEB D; 3IFD A; 3H3V C; 3QRF F; 2EY2 A; 3PUX A; 4XTC S; 1G91 A; 4Y15 A; 1BNZ A; 4L9J A; 5G3L F; 2GZW A; 4QB4 A; 3MZH A; 4PMC A; 3LA7 A; 2EXZ A; 3L9A X; 2RBM A; 1HTL D; 2PEX A; 4BAY A; 1T2K A; 4FNF A; 2CG4 A; 3QU6 A; 1WRJ A; 4BHP A; 3VYU A; 2N88 A; 5ILU A; 2EJM A; 2B2Y A; 4LAA A; 1X3Q A; 1ET9 A; 3R93 A; 1B44 D; 1A6X A; 3VYR A; 2IJ0 A; 4A3E B; 1R4Q B; 5T1I A; 5C3W A; 2CHB D; 1ONL A; 3JTG A; 1NSN S; 4CGZ A; 2K5W A; 1KQ0 A; 4HDV A; 4RHR A; 5IGG A; 2J5O A; 1SAX A; 1F9R A; 2MPM A; 5LGU A; 1DZ1 A; 2RSN A; 4EM2 A; 4XSZ F; 2K5Q A; 1SEB D; 2DY7 A; 5HOO A; 2EY6 A; 1AFP A; 2RV8 A; 3VB2 A; 2XV4 S; 4ENJ A; 1XDU A; 3MEH A; 4DGZ A; 1S5D D; 3KE2 A; 2XAF A; 2A61 A; 1EEF D; 1XGS A; 3PIO 3; 4ESB A; 3E5Q A; 4FT8 A; 2DK8 A; 4BQA A; 1FX7 A; 2QYO A; 2PN6 A; 1LLR D; 2GXB A; 3A8J E; 1XSV A; 1GVJ A; 1T5E A; 3W3A A; 3IFT A; 2XRO A; 4KJO A; 4WCG A; 4U0Y A; 3EYI A; 3D4D A; 4V19 0; 2A6E F; 3O2P E; 1O3Q A; 3FDT A; 1OMI A; 3C2P A; 2DI3 A; 4KHZ A; 2ZXR A; 4CXF A; 4KI0 A; 4YFX F; 1YFH A; 4A2U A; 1SE2 A; 2STW A; 3MCZ A; 3NK9 A; 3F8F A; 2FML A; 4S3S A; 4UV9 A; 2DY8 A; 2CWE A; 3WE2 A; 4TKO B; 3O13 A; 1G2T A; 1PZJ D; 2RVL A; 3QAH A; 3QIA A; 4IN3 A; 3COA C; 1R7J A; 5IGC A; 2LKP A; 1B1Z A; 2Z8L A; 2ZKZ A; 3SQN A; 3WGH A; 4B08 A; 4RGN A; 2VXW A; 2QWW A; 3O3K A; 2PCF B; 5KNC A; 2IT1 A; 4HBL A; 5EEH A; 3GD7 A; 2OZ6 A; 2XN9 C; 3WDN A; 3KUP A; 3TME A; 3T51 B; 4FYD A; 1T0F A; 2A68 F; 2EA4 A; 2EYF A; 1J9I A; 3R61 A; 3V2T A; 5C4H A; 5AIQ A; 4P96 A; 4HZF A; 3N52 A; 1RHP A; 4XQQ A; 1B4A A; 3PO2 B; 2M2L A; 1I50 B; 4HLX A; 2R3S A; 2B39 A; 3RI4 A; 1EY5 A; 3QB3 A; 4QIW B; 5HDG A; 2K03 A; 1R4P B; 1SNO A; 5KRU A; 1DOM A; 4EMS A; 1STE A; 2B8F A; 1ZH5 A; 3QWN A; 1STN A; 2ZNZ A; 3NP8 A; 3LFK A; 2ZBK A; 4PGG A; 4H7B A; 4IPA A; 2Y0S B; 3VYT A; 1AZP A; 2QDB A; 5KIX A; 3BJA A; 2EJR A; 5CR3 A; 3WTX C; 1HFG A; 2K32 A; 3MVV A; 4HAE A; 4ZLT F; 1EY0 A; 1RD9 D; 3FZV A; 1JOQ A; 1FP2 A; 5LEK A; 4IFD G; 3HUG A; 3K1N A; 3OHX C; 1XX8 A; 3M1E A; 4Q5S F; 2ESN A; 1SD7 A; 3T0Y A; 1PP8 F; 4XIG S; 4C3I B; 5FO9 B; 2LY7 A; 1UST A; 3OOC A; 3HRS A; 1LTB D; 1VTN C; 1PZI D; 4F8M A; 5M5Y B; 3KFW X; 5E1W A; 2FA5 A; 1NR4 A; 1SFO B; 1FY7 A; 3SVM A; 4C3H B; 4RGO S; 2VBX A; 4ZYG A; 2IJL A; 3K0L A; 2NWG A; 3NPP A; 5J8F A; 5D3I B; 4RAZ A; 2E1N A; 2LMC B; 1V8Q A; 4RCO A; 2JRT A; 2HFH A; 4K5W A; 3TDQ A; 1I6H B; 3N97 A; 1K8M A; 4OIP F; 4KHV A; 4RGT A; 3CUQ C; 3LWE A; 1ROD A; 2Q8R E; 4Q48 A; 1KU2 A; 3BZ6 A; 1BCP D; 2BDO A; 3I4M B; 2F0O A; 3F22 A; 1G6N A; 2HKO A; 4A3G B; 1EV7 A; 1QB5 D; 2K04 A; 3R2T A; 5C0X I; 1QZZ A; 5E20 A; 1SJE D; 1ZGJ A; 1PTO H; 3J80 Z; 1F9N A; 3H3U A; 1H9S A; 4ZYH A; 2M45 A; 2RKS A; 1H9S B; 4Z2Y A; 5L3F A; 4JQF A; 1YG2 A; 1H9R A; 2HDM A; 3FX3 A; 3E97 A; 3DV8 A; 4HQE A; 2NVQ B; 3TZU A; 5D4R A; 1F2M A; 3NTH A; 5E4W C; 1F77 A; 1SMT A; 5CV5 A; 4ZUJ A; 3R2I A; 3T1B A; 3GV6 A; 2W25 A; 2XRN A; 1HHV A; 4IUV A; 3GEZ A; 5ELB A; 3TO7 A; 2EJG C; 2PFB A; 3N6R A; 2B2U A; 4KIB A; 1IL8 A; 3L7W A; 2D5D A; 2QZ8 A; 1K79 A; 4LFU A; 5T1G A; 3J80 L; 1UEA B; 2F0N A; 1KTK A; 1Q1E A; 3F23 A; 1D5V A; 2B2V C; 3A7L A; 5AIP A; 3K0Y A; 2K02 A; 5HLI A; 4WXC A; 5EKL A; 1BCP C; 2Q8T A; 3GFM A; 1L0X B; 4EJV A; 5IIF A; 2F0E A; 5LEJ A; 5M3M B; 2F3W A; 2OZU A; 1PYW D; 1I3Q B; 3J7A O; 4A3J B; 4GCV A; 3J80 E; 1S5F D; 5D3D A; 1CZW A; 2A5Y B; 5BPD A; 2DU9 A; 3ZN0 A; 3F8M A; 5H6R A; 2DNC A; 5I9O A; 3MFK A; 4KN7 X; 1ESF A; 4A3M B; 2XAG A; 1EH7 A; 4XQJ A; 5J6X A; 3HRU A; 1FPQ A; 2DQR A; 3PIP 3; 2XVS A; 4IHS A; 5J39 A; 3F3X A; 1JOR A; 2PYK A; 5H6Q A; 3HTS B; 3A7A B; 3RLF A; 3TOB A; 1JWM D; 3WE3 A; 1OXX K; 2FQ3 A; 4RB1 A; 2IT0 A; 3SK4 A; 4TQU S; 1R9T B; 4L9V A; 3GV3 A; 3M4Y A; 3HOW B; 2M5W A; 1B4O A; 2J0T D; 4CKE A; 3BVG A; 5I2X A; 4K6L A; 5JHU A; 5FOB B; 3PUZ A; 2YX4 A; 1GUN A; 1Q12 A; 1DCZ A; 1MGT A; 3MXU A; 2MLK A; 5FFX A; 2EA2 A; 2KRF A; 3QPT A; 2IW3 A; 3A55 A; 1BI3 A; 1H0M A; 4DAY A; 2OQR A; 4R8N A; 1V43 A; 5IOC A; 2QVO A; 2HUG A; 2ELH A; 2GA2 A; 2MLG A; 1IF1 A; 4KN4 X; 1XD7 A; 1YW8 A; 2F0J A; 4ZYS A; 3R0A A; 4U68 A; 3L00 A; 2VN2 A; 3MEX A; 2M30 A; 3DQV C; 4UVC A; 4IXA A; 2GWR A; 2ID0 A; 5JOB A; 3KP6 A; 3K7A B; 4L0Z B; 3VA7 A; 3EYY A; 3I70 A; 4FO2 A; 3GTO B; 3WTZ A; 2X6L A; 5EEG A; 3BDO A; 2VC0 A; 1JHH A; 1USS A; 3DHQ A; 1YLF A; 2RDP A; 2P5L C; 2XSC A; 4OGQ C; 1ZEQ X; 4QMG A; 4A3I B; 1Y1V B; 3T8T A; 4Y17 A; 4LG0 A; 1WTX A; 4RA8 A; 5BN3 A; 5CLS A;
#similar chains in the Genus database (?% sequence similarity) ...loading similar chains, please wait... #similar chains, but unknotted ...loading similar chains, please wait... #similar chains in the pdb database (?% sequence similarity) ...loading similar chains, please wait...